deoxyribonucleic acid → dezoksiribonukleinska kiselina
Deoxyribonucleic acid (DNA) is a nucleic acid with 2-deoxy-D-ribose as the sugar in its nucleotides. DNA contains encoded genetic information, specifically templates for the synthesis of all of an organism’s proteins and enzymes.
DNA was first identified in the 1869 by Swiss chemist Friedrich Miescher (1844-1895). In 1953, American biologist James Dewey Watson (1928-) and English physicist Francis Harry Compton Crick (1916–2004) had discovered that DNA occurs in the cell as a double helix, with two long strands of the molecule wound around each other, and further that the chemical structure of the molecule dictates that adenine (A) always aligns or pairs with thymine (T), and cytosine (C) always pairs with guanine (G). It is this base pairing that allows DNA in a cell to copy itself, and transfer its information to a new cell. The diameter of the helix is 2.0 nm and there is a residue on each chain every 0.34 nm in the z direction. The angle between each residue on the same strand is 36°, so that the structure repeats after 10 residues (3.4 nm) on each strand.
disaccharide → disaharid
Disaccharides are compounds in which two monosaccharides are joined by a glycosidic bond. A glycosidic bond to the anomeric carbon can be either α or β. For example, maltose, the disaccharide obtained by enzyme-catalyzed hydrolysis of starch, consists of two D-glucopyranose units joined by a 1,4’-α-glycoside bond. The "prime" superscript indicates that C-4 is not in the same ring as C-1. Unlike the other disaccharides, sucrose is not a reducing sugar and does not exhibit mutarotation because the glycosidic bond is between the anomeric carbon of glucose and the anomeric carbon of fructose.
Fehling’s test → Fehlingova otopina
Fehling’s test is a chemical test to detect reducing sugars and aldehydes in a solution, devised by the German chemist Hermann Christian von Fehling (1812-1885). Fehling’s solution consists of Fehling’s A (copper(II) sulphate solution) and Fehling’s B (sodium tartarate solution), equal amounts of which are added to the test solution. After boiling, a positive result is indicated by the formation of a brick-red precipitate of copper(I) oxide. Methanal, being a strong reducing agent, also produces copper metal; ketones do not react. The test is now rarely used, having been replaced by Benedict’s test.
fructose → fruktoza
Fructose (fruit sugar) is a ketohexose (a six-carbon ketonic sugar), which occurs in sweet fruits and honey. Glucose and fructose have the same molecular formula, C6H12O6, but have different structures. Pure, dry fructose is a very sweet, white, odorless, crystalline solid. Fructose is one of the sweetest of all sugars and is combined with glucose to make sucrose, or common table sugar. An older common name for fructose is levulose, after its levorotatory property of rotating plane polarized light to the left (in contrast to glucose which is dextrorotatory). The polysaccharide inulin is a polymer of fructose.
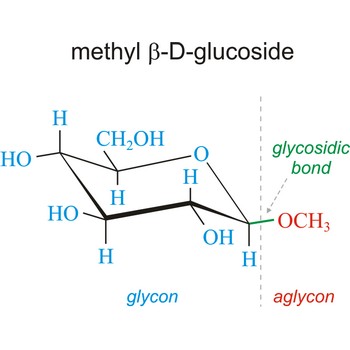
glycosidic bond → glikozidna veza
Glycosidic bond ia a bond between the glycosyl group, the structure obtained by removing the hydroxy group from the hemiacetal function of a monosaccharide, and the -OR group (which itself may be derived from a saccharide and chalcogen replacements thereof (RS–, RSe–). The terms N-glycosides and C-glycosides are misnomers and should not be used. The glycosidic bond can be α or β in orientation, depending on whether the anomeric hydroxyl group was α or β before the glycosidic bond was formed and on the specificity of the enzymatic reaction catalyzing their formation. Once the glycosidic bond is formed, the anomeric configuration of the ring is locked as either α or β. Specific glycosidic bonds therefore may be designated α(1→4), β(1→4), α(1→6), and so on. Cellulose is formed of glucose molecules linked by β(1→4)-glycosidic bonds, whereas starch is composed of α(1→4)-glycosidic bonds.
Citing this page:
Generalic, Eni. "Invertni šećer." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table