sacrificial protection → zaštita žrtvovanom elektrodom
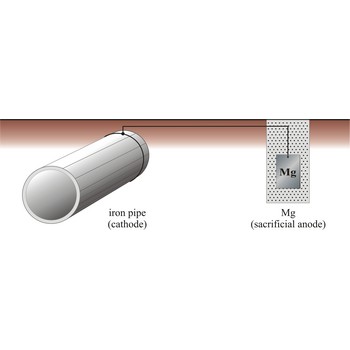
Sacrificial protection is the protection of iron or steel against corrosion by using a more reactive metal. Pieces of zinc or magnesium alloy are attached to pump bodies and pipes. The protected metal becomes the cathode and does not corrode. The anode corrodes, thereby providing the desired sacrificial protection. These items are known as sacrificial anodes and "attract" the corrosion to them rather than the iron/steel. The sacrificial anodes must be replaced periodically as they corrode.
The iron pipe will be connected to a more reactive metal such as magnesium through cooper wires, the magnesium will donate its electrons to the iron preventing it from rusting. Iron which is oxidises will immediately be reduced back to iron.
salt bridge → solni most
Salt bridge is a permeable material soaked in a salt solution that allows ions to be transferred from one container to another. The salt solution remains unchanged during this transfer.
salt water → slana voda
Salt water is the water of the sea and the ocean. This water contains a relatively high percentage of dissolved salt (about 35 g of salt per 1 000 g of sea water.). About 90 % of that salt would be sodium chloride, or ordinary table salt.
The salinity of ocean water varies. It is affected by such factors as melting of ice, inflow of river water, evaporation, rain, etc.
saturated fat → zasićena mast
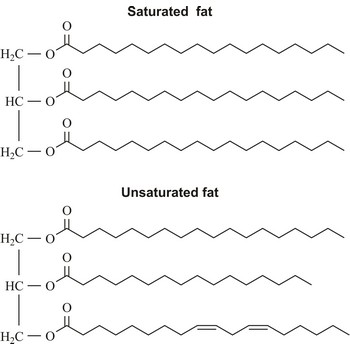
Saturated fats are fats in foods that are solid at room temperature. They come chiefly from animal sources (beef, whole-milk dairy products, dark meat poultry) but also from tropical vegetable oils (coconut, palm).
saturated fatty acid → zasićena masna kiselina
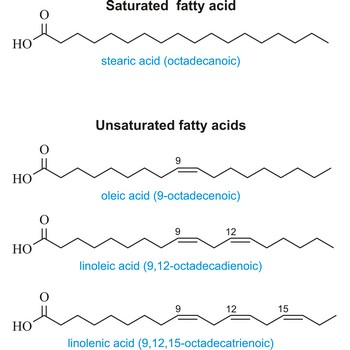
Saturated fatty acid is a fatty acid carrying the maximum possible number of hydrogen atoms (It doesn’t have any double bounds in the alkyl chain). The most important of these are:
| Butyric (butanoic acid) | CH3(CH2)2COOH |
| Lauric (dodecanoic acid) | CH3(CH2)10COOH |
| Myristic (tetradecanoic acid) | CH3(CH2)12COOH |
| Palmitic (hexadecanoic acid) | CH3(CH2)14COOH |
| Stearic (octadecanoic acid) | CH3(CH2)16COOH |
| Arachidic (eicosanoic acid) | CH3(CH2)18COOH |
Schellbach’s burette → Schellbachova bireta
When colourless liquids are used, parallax mistake is avoided by use of Schellbach’s burette. On the inside wall opposite to graduation scale it has a melted in ribbon from milky glass in the middle of which a blue line is found. The level of liquid is now spotted very easily because of light breaking in the meniscus blue line now looks like a double spike.
seawater → more
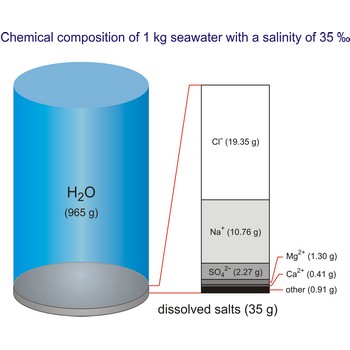
Seawater is a complex mixture of 96.5 % water, 3.5 % salts, and smaller amounts of other substances, including dissolved inorganic and organic materials, particulates, and a few atmospheric gases. The world's oceans cover nearly 71 % (361 840 000 km2) of the Earth's surface (510 100 000 km2), with an average depth of 3 682.2 m.
The density of seawater is higher than that of fresh water because of its higher salinity. Seawater's freezing point is lower than that of pure water and its boiling point is higher. The average salinity of the ocean is 35 ‰, which means that for every kilograms of water, there are 35 g of salt. The relative abundance of the major salts in seawater are constant regardless of the ocean. Only six elements and compounds comprise about 99 % of sea salts: chlorine (Cl-), sodium (Na+), sulfur (SO42-), magnesium (Mg2+), calcium (Ca2+), and potassium (K+).
sedimentation → sedimentiranje
Sedimentation is a process of separating specifically heavier, suspended matter, than the solution is. Solid matter settles on the bottom of the vessel and the liquid above it is poured off. The settling zone is the largest portion of the sedimentation basin. This zone provides the calm area necessary for the suspended particles to settle. The sludge zone, located at the bottom of the tank, provides a storage area for the sludge before it is removed for additional treatment or disposal.
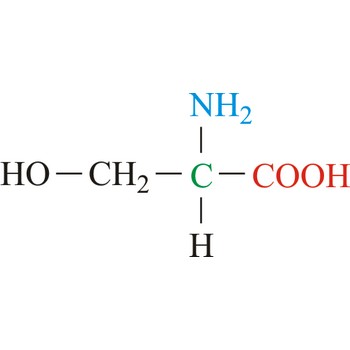
serine → serin
Serine is neutral amino acids with polar side chains. It is one of two hydroxyl amino acids. Both are commonly considered to by hydrophilic due to the hydrogen bonding capacity of the hydroxyl group. Serine often serves as a nucleophile in many enzyme active sites, and is best known for its role in the serine proteases. Serine is a site of phosphorylation and glycosylation which is important for enzyme regulation and cell signaling. It is not essential to the human diet, since it is synthesized in the body from other metabolites, including glycine.
- Abbreviations: Ser, S
- IUPAC name: 2-amino-3-hydroxypropanoic acid
- Molecular formula: C3H7NO3
- Molecular weight: 105.09 g/mol
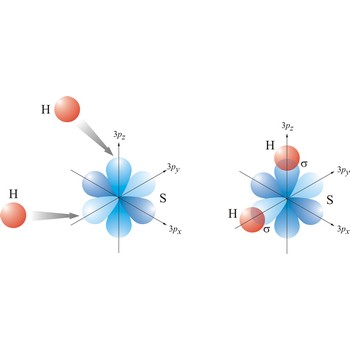
sigma bond → sigma veza
Most single bonds are sigma bonds (σ-bond). In the valence bond theory, a sigma bond is a valence bond that is symmetrical around the imaginary line between the bonded atoms.
Citing this page:
Generalic, Eni. "Visoka peć." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table