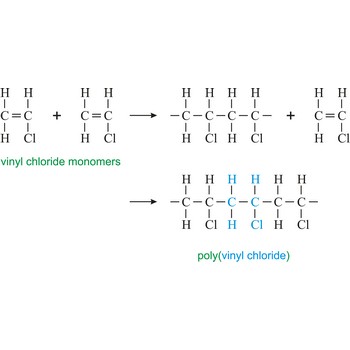
polyvinyl chloride → polivinil klorid
Poly(vinyl chloride) or the PVC is hard and resistant homopolymer produced by the polymerization of the gas vinyl chloride [CH2CHCl]. The pure polymer is hard, brittle and difficult to process, but it becomes flexible when plasticizers are added. After mixing with plasticizers, stabilizers, and pigments, the resin may be fabricated by techniques such as calendering, molding, or extrusion into flexible articles such as raincoats, shower curtains, and packaging films. The resin is not plasticized for use in making rigid products such as water pipe, plumbing fittings, and phonograph records.
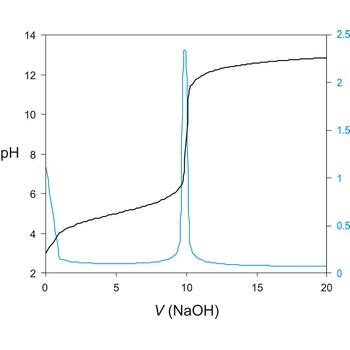
potentiometric titration → potenciometrijska titracija
Potentiometric titration is a volumetric method in which the potential between two electrodes is measured (referent and indicator electrode) as a function of the added reagent volume. Types of potentiometric titrations for the determination of analytes in photoprocessing solutions include acid-base, redox, precipitation, and complexometric.
Potentiometric titrations are preferred to manual titrations, since they are more accurate and precise. They are also more easily adapted to automation, where automated titration systems can process larger volumes of samples with minimal analyst involvement.
A titration curve has a characteristic sigmoid curve. The part of the curve that has the maximum change marks the equivalence point of the titration. The first derivative, ΔE/ΔV, is the slope of the curve, and the endpoint occurs at the volume, V', where ΔE/ΔV has the maximum value.
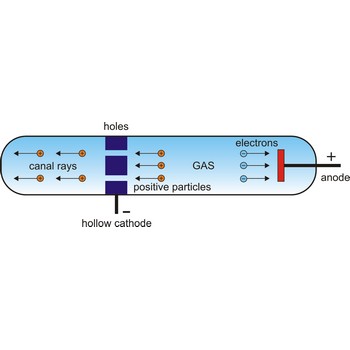
proton → proton
Proton is a stable elementary particle of unit positive charge and spin 1/2. Protons and neutrons, which are collectively called nucleons, are the constituents of the nucleus.
In 1886, German physicist Eugene Goldstein (1850-1930) discovered positive particles by using a modified Crookes tube with holes in the cathode in an evacuated tube. When cathode rays were given off in one direction toward the anode, other rays found their way through the holes in the cathode and sped off in the opposite direction. Since these other rays traveled in the direction opposite to the negatively charged cathode rays, it seemed that they must be composed of positively charged particles. Rutherford suggested that this fundamental positive particle be called the proton.
retardation factor → faktor zaostajanja
Retardation factor, RF, (in planar chromatography) is a ratio of the distance travelled by the centre of the spot to the distance simultaneously travelled by the mobile phase:
The RF value is characteristic for any given compound on the same stationary phase using the same mobile phase for development of the plates. Hence, known RF values can be compared to those of unknown substances to aid in their identifications.
ribonucleic acid → ribonukleinska kiselina
Ribonucleic acid is a complex organic compound in living cells that is concerned with protein synthesis. Plays an intermediary role in converting the information contained in DNA into proteins. RNA carries the genetic information from DNA to those parts of the cell where proteins are made. Some viruses store their genetic information as RNA not as DNA.
Ribonucleic acid is a similar molecule to DNA but with a slightly different structure.
The structural difference with DNA is that RNA contains a -OH group both at the 2' and 3' position of the ribose ring, whereas DNA (which stands, in fact, for deoxy-RNA) lacks such a hydroxy group at the 2' position of the ribose. The same bases can be attached to the ribose group in RNA as occur in DNA, with the exception that in RNA thymine does not occur, and is replaced by uracil, which has an H-group instead of a methyl group at the C-5 position of the pyrimidine. Unlike the double-stranded DNA molecule, RNA is a single-stranded molecule.
The three main functionally distinct varieties of RNA molecules are: (1) messenger RNA (mRNA) which is involved in the transmission of DNA information, (2) ribosomal RNa (rRNA) which makes up the physical machinery of the synthetic process, and (3) transfer RNA (tRNA) which also constitutes another functional part of the machinery of protein synthesis.
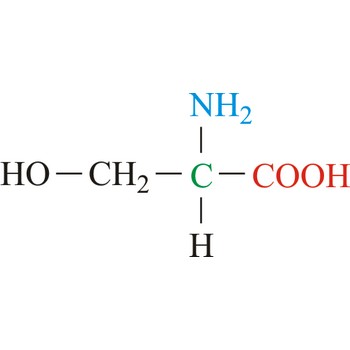
serine → serin
Serine is neutral amino acids with polar side chains. It is one of two hydroxyl amino acids. Both are commonly considered to by hydrophilic due to the hydrogen bonding capacity of the hydroxyl group. Serine often serves as a nucleophile in many enzyme active sites, and is best known for its role in the serine proteases. Serine is a site of phosphorylation and glycosylation which is important for enzyme regulation and cell signaling. It is not essential to the human diet, since it is synthesized in the body from other metabolites, including glycine.
- Abbreviations: Ser, S
- IUPAC name: 2-amino-3-hydroxypropanoic acid
- Molecular formula: C3H7NO3
- Molecular weight: 105.09 g/mol
solar cell → sunčeva ćelija
Solar cell, or photovoltaic cell, is a device that captures sunlight and transforms it directly to electricity. All solar cells make use of photovoltaic effect, so often they are called photovoltaic cells. Almost all solar cells are built from solid-state semiconducting materials, and in the vast majority of these the semiconductor is silicon.
The photovoltaic effect involves the generation of mobile charge carriers-electrons and positively charged holes-by the absorption of a photon of light. This pair of charge carriers is produced when an electron in the highest filled electronic band of a semiconductor (the valence band) absorbs a photon of sufficient energy to promote it into the empty energy band (the conduction band). The excitation process can be induced only by a photon with an energy corresponding to the width of the energy gap that separates the valence and the conduction band. The creation of an electron-hole pair can be converted into the generation of an electrical current in a semiconductor junction device, wherein a layer of semiconducting material lies back to back with a layer of either a different semiconductor or a metal. In most photovoltaic cells, the junction is p-n junction, in which p-doped and n-doped semiconductors are married together. At the interface of the two, the predominance of positively charged carriers (holes) in the p-doped material and of negatively charged carriers (electrons) in the n-doped material sets up an electric field, which falls off to either side of the junction across a space-charge region. When absorption of a photon in this region generates an electron-hole pair, these charge carriers are driven in opposite directions by the electric field, i.e. away from the interface and toward the top and bottom of the two-layer structure, where metal electrodes on these faces collect the current. The electrode on the top layer (through which light is absorbed) is divided into strips so as not to obscure the semiconducting layers below. In most widely used commercial solar cells, the p-doped and n-doped semiconductive layers are formed within a monolithic piece of crystalline silicon. Silicon is able to absorb sunlight at those wavelengths at which it is most intense-from the near-infrared region (wavelengths of around 1200 nm) to the violet (around 350 nm).
state of matter → agregatno stanje
State of matter is one of the tree physical states in which matter can exist, i.e. solid, liquid or gas. Plasma is sometimes regarded as the fourth state of matter. By means of heating a solid substance will cross to liquid state at its melting point. If we heat up a liquid and beyond, at its boiling point it will cross to gaseous state - vapour.
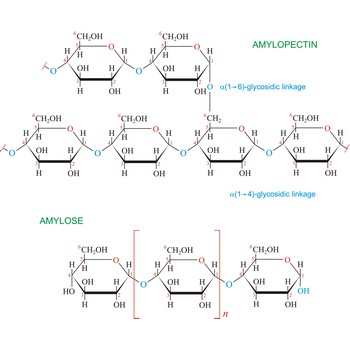
starch → škrob
Starch (C6H10O5)x is a polysaccharide used by plants to stockpile glucose molecules. It is the major component of flour, potatoes, rice, beans, corn, and peas. Starch is a mixture of two different polysaccharides: amylose (about 20 %), which is insoluble in cold water, and amylopectin (about 80 %), which is soluble in cold water. Amylose is composed of unbranched chains of D-glucose units joined by α(1→4)-glycosidic linkages. Unlike amylose, which are linear polymers, amylopectin contains α(1→6)-glycoside branches approximately every 25 glucose units.
Starch digestion begins in the mouth via the action of amylase, a digestive enzyme present in saliva. The process is completed in the small intestine by the pancreatic amylase. The final products of starch digestion, glucose molecules, are absorbed into the intestinal bloodstream and transported to the liver. Like most enzymes, glycosidases are highly selective in their action. They hydrolyze only the α-glycoside links in starch and leave the β-glycoside links in cellulose untouched. Starch is important food stuff and is used in adhesives, and sizes, in laundering, pharmacy and medicine.
supercritical fluid → superkritični fluid
Supercritical fluid is any substance above its critical temperature and critical pressure (see phase diagram). It shows unique properties that are different from those of either gases or liquids under standard conditions. A supercritical fluid has both the gaseous property of being able to penetrate anything, and the liquid property of being able to dissolve materials into their components. Solublity increases with increasing density (i.e. with increasing pressure). An example of this is naphthalene which is practically insoluble in low pressure carbon dioxide. At 100 bar the solubility is 10 g/L and at 200 bar it is 50 g/L. Rapid expansion of supercritical solutions leads to precipitation of a finely divided solid.
Citing this page:
Generalic, Eni. "Gallery/images.php." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table