electron → elektron
The electron is an elementary particle with a negative electric charge of (1.602 189 2±0.000 004 6)×10-19 C and a mass of 1/1837 that of a proton, equivalent to (9.109 534±0.000 047)×10-31 kg.
In 1897 the British physicist Joseph John (J.J.) Thomson (1856-1940) discovered the electron in a series of experiments designed to study the nature of electric discharge in a high-vacuum cathode-ray tube. Thomson interpreted the deflection of the rays by electrically charged plates and magnets as evidence of bodies much smaller than atoms that he calculated as having a very large value for the charge to mass ratio. Later he estimated the value of the charge itself.
Electrons are arranged in from one to seven shells around the nucleus; the maximum number of electrons in each shell is strictly limited by the laws of physics (2n2). The outer shells are not always filled: sodium has two electrons in the first shell (2×12 = 2), eight in the second (2×22 = 8), and only one in the third (2×32 = 18). A single electron in the outer shell may be attracted into an incomplete shell of another element, leaving the original atom with a net positive charge. Valence electrons are those that can be captured by or shared with another atom.
Electrons can be removed from the atoms by heat, light, electric energy, or bombardment with high-energy particles. Decaying radioactive nuclei spontaneously emit free electrons, called β particles.
Fahrenheit scale → Fahrenheitova skala
Fahrenheit scale is the temperature scale in which 212 degrees is the boiling point of water and 32 degrees is the freezing point of water. The scale was invented in 1714 by the German physicist G.D. Fahrenheit (1686-1736).
32 °F = 0 °C
212 °F = 100 °C
1 °F =(5/9) °C
T(°C) = (5/9)[T(°F) - 32]
T(°F) = (9/5)T(°C) + 32
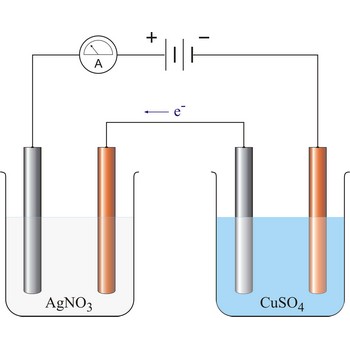
Faraday’s laws of electrolysis → Faradayevi zakoni elektrolize
Faraday’s laws of electrolysis are two laws found by British chemist and physicist Michael Faraday (1791-1867) in his experiments on electrolysis:
1. The quantity of matter extracted on the electrode is proportional to the quantity of charge (Q = I·t) which has flown in electrolysis time.
where z = number of electrons changed in reaction and F = Faraday’s constant which equals 96 487 C mol-1.
2. The masses of the elements liberated by the same quantity of electricity are directly proportional to their chemical equivalents.
96 487 C will discharge 1 mol Ag and 1/2 mol Cu. The relevant half reactions are:
fatty acid → masna kiselina
Fatty acids are aliphatic monocarboxylic acids characterized by a terminal carboxyl group (R-COOH). The higher members of this series of acids occur in nature in the combined form of esters of glycerol (fats), and hence all acids of this family are called fatty acids. Natural fatty acids commonly have a chain of 4 to 28 carbons (usually unbranched and even-numbered), which may be saturated or unsaturated. The most important of saturated fatty acids are butyric (C4), lauric (C12), palmitic (C16), and stearic (C18). The most common unsaturated acids are oleic, linoleic, and linolenic (all C18).
The physical properties of fatty acids are determined by the chain length, degree of unsaturation, and chain branching. Short-chain acids are pungent liquids, soluble in water. As the chain length increases, melting points are raised and water-solubility decreases. Unsaturation and chain branching tend to lower melting points.
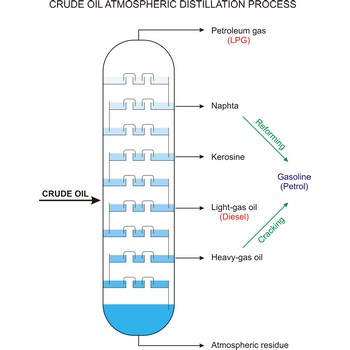
fractional distillation → frakcijska destilacija
Fractional distillation is a procedure in which liquids of close boiling points are separated. It is conducted in fraction or rectification columns in a way that vapour phase created by distillation is condensed and the condensate thus obtained is redistilled. The procedure is repeated several times. Vapour phase always contains more volatile component than the liquid phase, at top of the column vapour of clean volatile component gets out and at the bottom of the column liquid of nonvolatile component.
glutamic acid → glutaminska kiselina
Glutamic acid is an electrically charged amino acids. It is one of the two amino acids that contain a carboxylic acid group in its side chains. These acids play important roles as general acids in enzyme active centers, as well as in maintaining the solubility and ionic character of proteins. Glutamic acid is commonly referred to as glutamate, because its carboxylic acid side chain will be deprotonated and thus negatively charged in its anionic form at physiological pH. Glutamic acid is referred to as a non-essential amino acid because a healthy human can synthesize all the glutamic acid needed for normal body function from other amino acids.
- Abbreviations: Glu, E
- IUPAC name: 2-aminopentanedioic acid
- Molecular formula: C5H9NO4
- Molecular weight: 147.13 g/mol
glucose → glukoza
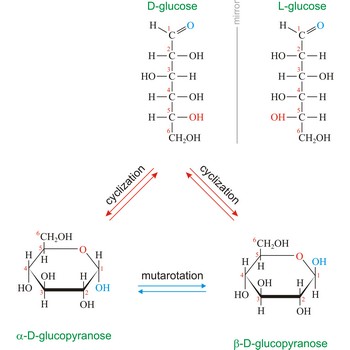
Glucose (grape sugar, blood sugar), C6H12O6, is an aldohexose (a monosaccharide sugar having six carbon atoms and an aldehyde group). An older common name for glucose is dextrose, after its dextrorotatory property of rotating plane polarized light to the right. Glucose in free (in sweet fruits and honey) or combined form (sucrose, starch, cellulose, glycogen) is is probably the most abundant organic compound in nature. During the photosynthesis process, plants use energy from the sun, water from the soil and carbon dioxide gas from the air to make glucose. In cellular respiration, glucose is ultimately broken down to yield carbon dioxide and water, and the energy from this process is stored as ATP molecules (36 molecules of ATP across all processes).
Naturally occurring glucose is D isomers (OH group on the stereogenic carbon farthest from the aldehyde group, C-5, is to the right in the Fischer projection). Although often displayed as an open chain structure, glucose and most common sugars exist as ring structures. In the α form, the hydroxyl group attached to C-1 and the CH2OH attached to C-5 are located on opposite sides of the ring. β-glucose has these two groups on the same side of the ring. The full names for these two anomers of glucose are α-D-glucopyranose and β-D-glucopyranose.
glycoside → glikozid
Glycoside is one of a group of organic compounds in which a sugar group is bonded through its anomeric carbon to another group via a glycosidic bond. The sugar group is known as the glycon and the non-sugar group as the aglycon. According to the IUPAC definition, all disaccharides and polysaccharides are glycosides where the aglycone is another sugar.
In the free hemiacetal form, sugars will spontaneously equilibrate between the α and β anomers. However, once the glycosidic bond is formed, the anomeric configuration of the ring is locked as either α or β. Therefore, the alpha and beta glycosides are chemically distinct. They will have different chemical, physical, and biological properties. Many glycosides occur abundantly in plants, especially as flower and fruit pigments.
The term glycoside was later extended to cover not only compounds in which the anomeric hydroxy group is replaced by a group -OR, but also those in which the replacing group is -SR (thioglycosides), -SeR (selenoglycosides), -NR1R2 (N-glycosides), or even -CR1R2R3 (C-glycosides). Thioglycoside and selenoglycoside are legitimate generic terms; however the use of N-glycoside, although widespread in biochemical literature, is improper and not recommended here (glycosylamine is a perfectly acceptable term). C-Glycoside is even less acceptable. All other glycosides are hydrolysable; the C-C bond of C-glycosides is usually not. The use and propagation of names based on C-glycoside terminology is therefore strongly discouraged.
Gratzel solar cell → Gratzelova sunčeva ćelija
Grätzel solar cell is photoelectrochemical cell, developed by Michael Grätzel and collaborators, simulates some characteristics of the natural solar cell, which enables photosynthesis take place. In natural solar cell the chlorophyll molecules absorb light (most strongly in the red and blue parts of the spectrum, leaving the green light to be reflected). The absorbed energy is sufficient to knock an electron from the excited chlorophyll. In the further transport of electron, other molecules are involved, which take the electron away from chlorophyll. In Grätzel cell, the tasks of charge-carrier generation and transport are also assigned to different species.
His device consists of an array of nanometre-sized crystallites of the semiconductor titanium dioxide, welded together and coated with light-sensitive molecules that can transfer electrons to the semiconductor particles when they absorb photons. So, light-sensitive molecules play a role equivalent to chlorophyll in photosynthesis. In Grätzel cell, the light-sensitive molecule is a ruthenium ion bound to organic bipyridine molecules, which absorb light strongly in the visible range; titanium dioxide nanocrystals carry the received photoexcited electrons away from electron donors. On the other hand, a donor molecule must get back an electron, so that it can absorb another photon. So, this assembly is immersed in a liquid electrolyte containing molecular species (dissolved iodine molecules) that can pick up an electron from an electrode immersed in the solution and ferry it to the donor molecule. These cells can convert sunlight with efficiency of 10 % in direct sunlight and they are even more efficient in diffuse daylight.
histidine → histidin
Histidine is an electrically charged amino acids with basic side chains. It is an essential amino acid, which means that humans cannot synthesize it, so it must be ingested. Histidine is perhaps the most common and versatile catalytic residue in proteins. The imidazole sidechain of histidine has a pKa of approximately 6.0. This means that, at physiologically relevant pH values, relatively small shifts in pH will change its average charge. The unprotonated imidazole is nucleophilic and can serve as a general base, while the protonated form can serve as a general acid. In addition, it is often a ligand for transition metal ions such as iron and zinc.
- Abbreviations: His, H
- IUPAC name: 2-amino-3-(1H-imidazol-5-yl)propanoic acid
- Molecular formula: C6H9N3O2
- Molecular weight: 155.15 g/mol
Citing this page:
Generalic, Eni. "Gallery/images.php." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table