atmosphere → atmosfera
1. Atmosphere is the column of air which is extending several hundred kilometers above the surface the Earth's surface. The density of this air decreases as you proceed up from the surface. The air in the atmosphere consists of 78 % nitrogen, 21 % oxygen, and 0.9 % argon. The remaining 0.1 % of the atmosphere consists of ozone, water vapor, carbon dioxide, methane, helium, and neon. The atmosphere is divided into different regions. The lowest two layers are the troposphere (the layer closest to the earth) and the stratosphere respectively. These two layers contain more than 99 % of the atmospheric molecules.
2. Standard atmosphere (atm) is an obsolete pressure and stress unit which should be discontinued. It is unit of pressure equal to the air pressure measured at mean sea level.
1 atm = 101 325 Pa
Technical atmosphere (at) is an obsolete MKpS pressure and sttress derived unit.
1 at = 98 066.5 Pa
1 atm = 1.033 227 453 at
carbonization → karbonizacija
Carbonization begins when you heat organic substances like wood, sugar or meat with no presence of air; they go black because of secreted carbon.
benzene → benzen
Benzene is a colourless liquid hydrocarbon, C6H6, b.p. 80 °C. It is now made from petroleum by catalytic reforming (formerly obtained from coal tar). Benzene is the archetypal aromatic compound. It has an unsaturated molecule, yet will not readily undergo addition reactions. On the other hand, it does undergo substitution reactions in which hydrogen atoms are replaced by other atoms or groups.
In 1865, Friedrich August Kekulé purposed the benzene molecule structure as a hexagonal ring which consists of six carbon atoms with alternate carbon-carbon single and carbon-carbon double bond. But such a structure should be highly reactive, and so didn't account for the unreactive nature of benzene. We now know that the best representation for the structure of benzene is indeed, hexagonal, with each C-C bond distance being identical and intermediate between those for a single and double bond. The π-orbitals from each neighbouring carbon atom overlap to form a delocalised molecular orbital which extends around the ring, giving added stability and with it, decreased reactivity. That is the reason the structural formula of benzene represents as a hexagon with a circle in the center which represents the delocalized electrons.
beta particle → beta-čestica
Beta particle is a charged particle emitted from a radioactive atomic nucleus either natural or manufactured. The energies of beta particles range from 0 MeV to 4 MeV. They carry a single charge; if this is negative, the particle is identical with an electron; if positive, it is a positron.
An unstable atomic nucleus changes into a nucleus of the same mass number but different proton number with the emission of an electron and an antineutrino (or a positron and a neutrino)
biocapacity → biokapacitet
Biocapacity (or biological capacity) is the capacity of ecosystems to produce useful biological materials and to absorb carbon dioxide generated by humans, using current management schemes and extraction technologies. Useful biological materials are defined as those used by the human economy, hence what is considered useful can change from year to year. The biocapacity of an area is calculated by multiplying the actual physical area by the yield factor and the appropriate equivalence factor.
Yield factor is a factor that accounts for differences between countries in productivity of a given land type. Each country and each year has yield factors for cropland, grazing land, forest, and fisheries.
Equivalence factor is a productivity based scaling factor that converts a specific land type into a universal unit of biologically productive area, a global hectare (gha).
catalytic hydrogenation → katalitičko hidrogeniranje
Catalytic hydrogenation is the infusing of unsaturated or impure hydrocarbons with hydrogen gas at controlled temperatures and pressures and in the presence of a catalyst for the purpose of obtaining saturated hydrocarbons and/or removing various impurities such as sulphur and nitrogen.
cementation → cementacija
Cementation is any metallurgical process in which the surfaces of a metal is impregnated by some other substance, especially an obsolete process for making steel by heating bars of wrought iron to red heat for several days in a bed of charcoal.
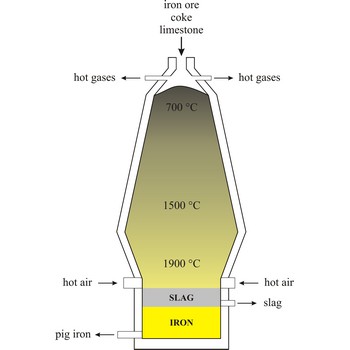
blast furnace → visoka peć
Blast furnace is a furnace for smelting of iron from iron oxide ores (hematite, Fe2O3 or magnetite, Fe3O4). Coke, limestone and iron ore are poured in the top, which would normally burn only on the surface. The hot air blast to the furnace burns the coke and maintains the very high temperatures that are needed to reduce the ore to iron. The reaction between air and the fuel generates carbon monoxide. This gas reduces the iron(III) oxide in the ore to iron.
Because the furnace temperature is in the region of 1500 °C, the metal is produced in a molten state and this runs down to the base of the furnace.
The production of iron in a blast furnace is a continuous process. The furnace is heated constantly and is re-charged with raw materials from the top while it is being tapped from the bottom. Iron making in the furnace usually continues for about ten years before the furnace linings have to be renewed.
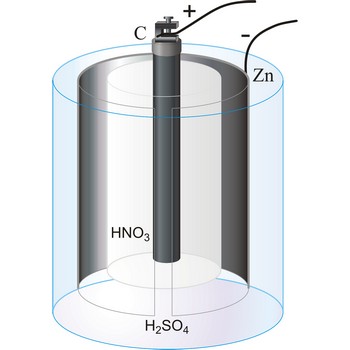
Bunsen’s cell → Bunsenov članak
Bunsen’s cell is a primary cell devised by Robert W. Bunsen consisting of a zinc cathode immersed in dilute sulphuric acid and carbon anode immersed in concentrated nitric acid. The electrolytes are separated by a porous pot. The cell gives an e.m.f. of about 1.9 V.
chemical property → kemijsko svojstvo
Chemical property is a property observed when a substance undergoes a transformation into one or more new substances. Measurement of a chemical property involves a chemical change. For example, determining the flammability of petrol involves burning it, producing carbon dioxide and water.
Citing this page:
Generalic, Eni. "Ugljik." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table