Geiger counter → Geigerov brojač
Geiger counter (Geiger-Muller counter) is a device used to detect and measure ionising radiation. It consists of a tube containing a low-pressure gas (usually argon or neon with methane) and a cylindrical hollow cathode through the centre of which runs a fine-wire anode. A potential difference of about 1 000 V is maintained between the electrodes. An ionising particle or photon passing through a window into the tube will cause an ion to be produced and the high potential will accelerate it towards its appropriate electrode, causing an avalanche of further ionisations by collision. The consequent current pulses can be counted in electronic circuits or simply amplified to work a small loudspeaker in the instrument. It was first devised in 1908 by the German physicist Hans Geiger (1882-1945). Geiger and W. Muller produced an improved design in 1928.
global warming → globalno zatopljenje
Global warming or greenhouse effect is an effect occurring in the atmosphere because of the presence of certain gases (greenhouse gases) that absorb infrared radiation. Light and ultraviolet radiation from the sun is able to penetrate the atmosphere and warm the Earth’s surface. This energy is re-radiated as infrared radiation which because of its longer wavelength, is absorbed by such substances as carbon dioxide. The overall effect is that the average temperature of the Earth and its atmosphere is increasing (so-called global Warming). The effect is similar to that occurring in a greenhouse, where light and long-wavelength ultraviolet radiation can pass through the glass into greenhouse but the infrared radiation is absorbed by the glass and part of it is re-radiated into the greenhouse.
The greenhouse effect is seen as a major environmental hazard. Average increases in temperature could change weather patterns and agricultural output. It might also lead to melting of the polar ice caps and a corresponding rise in sea level. Carbon dioxide, from fossil-fuel power stations and car exhausts, is the main greenhouse gas. Other contributory pollutants are nitrogen oxides, ozone, methane, and chloroflourocarbons.
petroleum ether → petroleter
Petroleum ether is the petroleum fraction consisting of C5 and C6 hydrocarbons and boiling in the range 35 °C to 60 °C; commonly used as a laboratory solvent.
potassium-argon dating → kalij-argonsko datiranje
Potassium-argon dating is a process of determining the age of mineral deposits by measuring the proportion between quantity of isotope 40Ar and 40K in mineral deposits.
glucose → glukoza
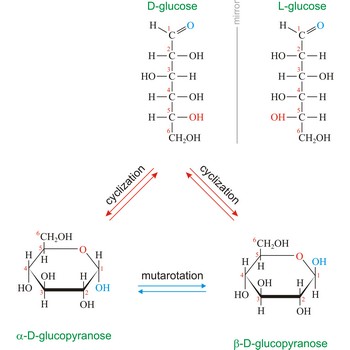
Glucose (grape sugar, blood sugar), C6H12O6, is an aldohexose (a monosaccharide sugar having six carbon atoms and an aldehyde group). An older common name for glucose is dextrose, after its dextrorotatory property of rotating plane polarized light to the right. Glucose in free (in sweet fruits and honey) or combined form (sucrose, starch, cellulose, glycogen) is is probably the most abundant organic compound in nature. During the photosynthesis process, plants use energy from the sun, water from the soil and carbon dioxide gas from the air to make glucose. In cellular respiration, glucose is ultimately broken down to yield carbon dioxide and water, and the energy from this process is stored as ATP molecules (36 molecules of ATP across all processes).
Naturally occurring glucose is D isomers (OH group on the stereogenic carbon farthest from the aldehyde group, C-5, is to the right in the Fischer projection). Although often displayed as an open chain structure, glucose and most common sugars exist as ring structures. In the α form, the hydroxyl group attached to C-1 and the CH2OH attached to C-5 are located on opposite sides of the ring. β-glucose has these two groups on the same side of the ring. The full names for these two anomers of glucose are α-D-glucopyranose and β-D-glucopyranose.
glycosidic bond → glikozidna veza
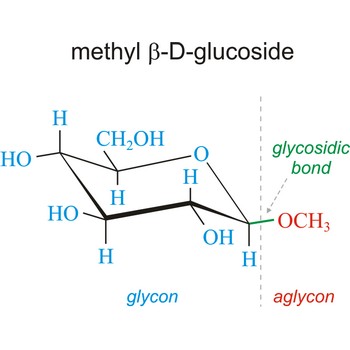
Glycosidic bond ia a bond between the glycosyl group, the structure obtained by removing the hydroxy group from the hemiacetal function of a monosaccharide, and the -OR group (which itself may be derived from a saccharide and chalcogen replacements thereof (RS–, RSe–). The terms N-glycosides and C-glycosides are misnomers and should not be used. The glycosidic bond can be α or β in orientation, depending on whether the anomeric hydroxyl group was α or β before the glycosidic bond was formed and on the specificity of the enzymatic reaction catalyzing their formation. Once the glycosidic bond is formed, the anomeric configuration of the ring is locked as either α or β. Specific glycosidic bonds therefore may be designated α(1→4), β(1→4), α(1→6), and so on. Cellulose is formed of glucose molecules linked by β(1→4)-glycosidic bonds, whereas starch is composed of α(1→4)-glycosidic bonds.
primary alcohol → primarni alkohol
Primary alcohols are alcohols where the hydroxyl group is attached to a primary carbon atom. Thus, it has the general structure, RCH2OH, where R is a hydrogen atom or an alkyl group.
Citing this page:
Generalic, Eni. "Određivanje starosti radioaktivnim ugljikom." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table