carboxylates → karboksilati
Carboxylates is a common name for all salts that carboxylic acids yield by reacting with hydroxides, carbonates, bicarbonates and other alkaline reagents.
saponification → saponifikacija
Saponification is a proces of hydrolysis of esters using hot sodium hydroxide solution to produce the salt of a carboxylic acid. Saponification usually refers to the hydrolysis of esters of fatty acids to manufacture soaps.
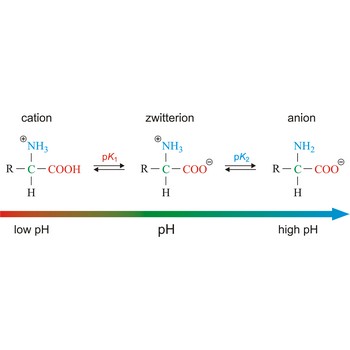
zwitterion → dipolarni ion
Zwitterion, also known as inner salt or dipolar ion, is an ion with a positive and a negative electrical charge at different locations within a molecule. As the molecule contains two opposite charges, it is electrically neutral. The term zwitterion is derived from the German word zwitter, meaning a hybrid, hermaphrodite. Zwitterions can be formed from compounds that contain both acid groups and base groups in their molecules (ampholytes).
All of the common amino acids found in proteins are ampholytes because they contain a carboxyl group (-COOH) that acts as an acid and an amino group (-NH2) that acts as a base. In the solid state, amino acids exist in the dipolar or zwitterion form. If acid is added to a solution containing the zwitterion, the carboxylate group captures a hydrogen (H+) ion, and the amino acid becomes positively charged. If base is added, ion removal of the H+ ion from the amino group of the zwitterion produces a negatively charged amino acid.
acid halide → kiselinski halogenid
Acid halide is organic compound containing the group -COX where X is a halogen atom.
acid radical → kiseli radikal
Acid radical is an anion left after removal of hydrogen atoms from an acid.
acid salt → kisela sol
Acid salt is a compound formed by replacing hydrogen in an acid with a metal (or a radical that acts like a metal).
acid-base indicator → kiselo-bazni indikator
Acid-base indicator is a weak acid or weak base, such as litmus, methyl orange or phenolphthalein, which changes colour when it gains or loses an H+ ion.
Citing this page:
Generalic, Eni. "Karboksilne kiseline." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table