carboxylic acids → karboksilne kiseline
Carboxylic acids are organic compounds characterized by the presence of one or more RC(=O)OH groups (the carboxyl group). In the systematic chemical nomenclature carboxylic acids names end in the suffix -oic (e.g. ethanoic acids, CH3COOH). The carbon of the terminal group being counted as part of the chain. They are generally weak acids. Carboxylic acids include a large and important class of fatty acids and may be either saturated or unsaturated. There are also some natural aromatic carboxylic acids (benzoic, salicylic).
acid dissociation constant → konstanta disocijacije kiseline
Acid dissociation constant (Ka) is the equilibrium constant for the dissociation of an acid HA through the reaction
The quantity pKa = -log Ka is often used to express the acid dissociation constant.
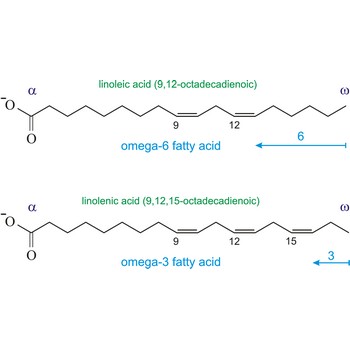
omega-3 fatty acids → omega-3 masne kiseline
Omega-3 fatty acids are polyunsaturated fatty acids, meaning they contain more than one double bond. The name omega-3 indicates that the first double bond occurs on the third carbon atom (n-3) from the methyl (-CH3) end of the molecule (omega position). The three main omega-3 fatty acids are alpha-linolenic acid (ALA, 18:3n-3), eicosapentaenoic acid (EPA, 20:5n-3), and docosahexaenoic acid (DHA, 22:6n-3). ALA comes from plants. EPA and DHA come from fish.
Similarly, the first double bond in omega-6 fatty acids is located between the sixth and seventh carbon atom (n-6) from the methyl end of the fatty acid (omega end).
polyprotonic acids → poliprotonske kiseline
Polyprotonic acids are acids which dissolve in more than one degree.
carboxylate group → karboksilna skupina
Carboxylate group (-COOH) is a functional group which is common to all carboxylic acids.
organic acid → organska kiselina
Organic acid is an organic compound which is sour, most often these are carboxylic acids (RCOOH).
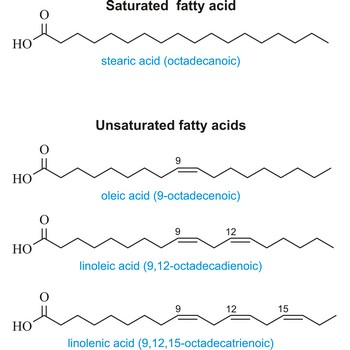
fatty acid → masna kiselina
Fatty acids are aliphatic monocarboxylic acids characterized by a terminal carboxyl group (R-COOH). The higher members of this series of acids occur in nature in the combined form of esters of glycerol (fats), and hence all acids of this family are called fatty acids. Natural fatty acids commonly have a chain of 4 to 28 carbons (usually unbranched and even-numbered), which may be saturated or unsaturated. The most important of saturated fatty acids are butyric (C4), lauric (C12), palmitic (C16), and stearic (C18). The most common unsaturated acids are oleic, linoleic, and linolenic (all C18).
The physical properties of fatty acids are determined by the chain length, degree of unsaturation, and chain branching. Short-chain acids are pungent liquids, soluble in water. As the chain length increases, melting points are raised and water-solubility decreases. Unsaturation and chain branching tend to lower melting points.
glutamic acid → glutaminska kiselina
Glutamic acid is an electrically charged amino acids. It is one of the two amino acids that contain a carboxylic acid group in its side chains. These acids play important roles as general acids in enzyme active centers, as well as in maintaining the solubility and ionic character of proteins. Glutamic acid is commonly referred to as glutamate, because its carboxylic acid side chain will be deprotonated and thus negatively charged in its anionic form at physiological pH. Glutamic acid is referred to as a non-essential amino acid because a healthy human can synthesize all the glutamic acid needed for normal body function from other amino acids.
- Abbreviations: Glu, E
- IUPAC name: 2-aminopentanedioic acid
- Molecular formula: C5H9NO4
- Molecular weight: 147.13 g/mol
acid → kiselina
Acid is a type of compound that contains hydrogen and dissociates in water to produce positive hydrogen ions. The reaction for an acid HA is commonly written:
In fact, the hydrogen ion (the proton) is solvated, and the complete reaction is:
This definition of acids comes from the Arrhenius theory. Such acids tend to be corrosive substances with a sharp taste, which turn litmus red and produce colour changes with other indicators. They are referred to as protonic acids and are classified into strong acids, which are almost completely dissociated in water, (e.g. sulphuric acid and hydrochloric acid), and weak acids, which are only partially dissociated (e.g. acetic acid and hydrogen sulphide). The strength of an acid depends on the extent to which it dissociates, and is measured by its dissociation constant.
In the Lowry-Brønsted theory of acids and bases (1923), the definition was extended to one in which an acid is a proton donor (a Brønsted acid), and a base is a proton acceptor (a Brønsted base). An important feature of the Lowry-Brønsted concept is that when an acid gives up a proton, a conjugate base is formed that is capable of accepting a proton.
Similarly, every base produces its conjugate acid as a result of accepting a proton.
For example, acetate ion is the conjugate base of acetic acid, and ammonium ion is the conjugate acid of ammonia.
As the acid of a conjugate acid/base pair becomes weaker, its conjugate base becomes stronger and vice versa.
A further extension of the idea of acids and bases was made in the Lewis theory. In this, a G. N. Lewis acid is a compound or atom that can accept a pair of electrons and a Lewis base is one that can donate an electron pair. This definition encompasses "traditional" acid-base reactions, but it also includes reactions that do not involve ions, e.g.
in which NH3 is the base (donor) and BCl3 the acid (acceptor).
acrylic acid → akrilna kiselina
Acrylic acid (propenoic acid) is a colourless liquid, smelling like acetic acid. It can be formed by acrolein oxidation. It readily polymerizes and is used in the manufacture of acrylic resins, transparent plastic materials (organic glass).
Citing this page:
Generalic, Eni. "Karboksilne kiseline." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table