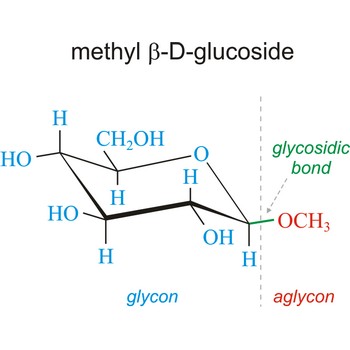
glycosidic bond → glikozidna veza
Glycosidic bond ia a bond between the glycosyl group, the structure obtained by removing the hydroxy group from the hemiacetal function of a monosaccharide, and the -OR group (which itself may be derived from a saccharide and chalcogen replacements thereof (RS–, RSe–). The terms N-glycosides and C-glycosides are misnomers and should not be used. The glycosidic bond can be α or β in orientation, depending on whether the anomeric hydroxyl group was α or β before the glycosidic bond was formed and on the specificity of the enzymatic reaction catalyzing their formation. Once the glycosidic bond is formed, the anomeric configuration of the ring is locked as either α or β. Specific glycosidic bonds therefore may be designated α(1→4), β(1→4), α(1→6), and so on. Cellulose is formed of glucose molecules linked by β(1→4)-glycosidic bonds, whereas starch is composed of α(1→4)-glycosidic bonds.
half-life → vrijeme poluraspada
For a simple radioactive decay process, half-life, t1/2, is defined as the time required for the activity of a given radioactive isotopes to decrease to half its value by that process.
The half-life is a characteristic property of each radioactive isotope and is independent of its amount or condition.
Gratzel solar cell → Gratzelova sunčeva ćelija
Grätzel solar cell is photoelectrochemical cell, developed by Michael Grätzel and collaborators, simulates some characteristics of the natural solar cell, which enables photosynthesis take place. In natural solar cell the chlorophyll molecules absorb light (most strongly in the red and blue parts of the spectrum, leaving the green light to be reflected). The absorbed energy is sufficient to knock an electron from the excited chlorophyll. In the further transport of electron, other molecules are involved, which take the electron away from chlorophyll. In Grätzel cell, the tasks of charge-carrier generation and transport are also assigned to different species.
His device consists of an array of nanometre-sized crystallites of the semiconductor titanium dioxide, welded together and coated with light-sensitive molecules that can transfer electrons to the semiconductor particles when they absorb photons. So, light-sensitive molecules play a role equivalent to chlorophyll in photosynthesis. In Grätzel cell, the light-sensitive molecule is a ruthenium ion bound to organic bipyridine molecules, which absorb light strongly in the visible range; titanium dioxide nanocrystals carry the received photoexcited electrons away from electron donors. On the other hand, a donor molecule must get back an electron, so that it can absorb another photon. So, this assembly is immersed in a liquid electrolyte containing molecular species (dissolved iodine molecules) that can pick up an electron from an electrode immersed in the solution and ferry it to the donor molecule. These cells can convert sunlight with efficiency of 10 % in direct sunlight and they are even more efficient in diffuse daylight.
halocarbon → halogenirani ugljikovodik
Halocarbon is a compound containing no elements other than carbon, one or more halogens, and sometimes hydrogen. The simplest are compounds such as tetrachloromethane (CCl4), tetrabromomethane (CBr4), etc. The lower members of the various homologous series are used as refrigerants, propellant gases, fireextinguishing agents, and blowing agents for urethane foams. When polymerized, they yield plastics characterized by extreme chemical resistance, high electrical resistivity, and good heat resistance.
halogens → halogeni elementi
Halogens are the elements fluorine (F) chlorine (Cl), bromine (Br), iodine (I), and astatine (At). They are non-metals, and make up part of the 17 group in the periodic table. Compounds of these elements are called halogenides or halides.
The halogens all have a strong unpleasant odour and will burn flesh. They do not dissolve well in water. The five elements are strongly electronegative. They are oxidising agents, with fluorine being the strongest and astatine being the weakest. They react with most metals and many non-metals.
Halogens form molecules which consist of atoms covalently bonded. With increasing atomic weight there is a gradation in physical properties. For example: Fluorine is a pale green gas of low density. Chlorine is a greenish-yellow gas 1.892 times as dense as fluorine. Bromine is a deep reddish-brown liquid which is three times as dense as water. Iodine is a grayish-black crystalline solid with a metallic appearance. And astatine is a solid with properties which indicate that it is somewhat metallic in character.
Henry’s law → Henryjev zakon
Henry’s law was discovered in 1801 by the British chemist William Henry (1775-1836). At a constant temperature the mass of gas dissolved in a liquid at equilibrium is proportional to the partial pressure of the gas. It applies only to gases that do not react with the solvent.
where pi is the partial pressure of component i above the solution, xi is its mole fraction in the solution, and Kx is the Henry’s law constant (a characteristic of the given gas and solvent, as well as the temperature).
histidine → histidin
Histidine is an electrically charged amino acids with basic side chains. It is an essential amino acid, which means that humans cannot synthesize it, so it must be ingested. Histidine is perhaps the most common and versatile catalytic residue in proteins. The imidazole sidechain of histidine has a pKa of approximately 6.0. This means that, at physiologically relevant pH values, relatively small shifts in pH will change its average charge. The unprotonated imidazole is nucleophilic and can serve as a general base, while the protonated form can serve as a general acid. In addition, it is often a ligand for transition metal ions such as iron and zinc.
- Abbreviations: His, H
- IUPAC name: 2-amino-3-(1H-imidazol-5-yl)propanoic acid
- Molecular formula: C6H9N3O2
- Molecular weight: 155.15 g/mol
Hesse’s law → Hessov zakon
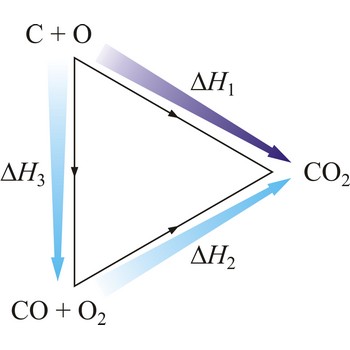
Hesse’s law says that reaction heat of some chemical change does not depend on the way in which the reaction is conducted, but only on starting and ending system state. Hesse’s law is also known as the law of constant heat summation. Hesse’s law is also known as the law of constant heat summation. The law was first put forward in 1840 by the Swiss-born Russian chemist Germain Henri Hess (1802-1850).
Hesse’s law can be used to obtain thermodynamic data that cannot be measured directly. For example, it is very difficult to control the oxidation of graphite to give pure CO. However, enthalpy for the oxidation of graphite to CO2 can easily be measured. So can the enthalpy of oxidation of CO to CO2. The application of Hess’s law enables us to estimate the enthalpy of formation of CO.
| C(s) + O2(g) →← CO2(g) | ΔrH1 = -393 kJ mol-1 |
| CO(g) + 1/2O2(g) →← CO2(g) | ΔrH2 = -283 kJ mol-1 |
| C(s) + 1/2O2(g) →← CO(g) | ΔrH3 = -110 kJ mol-1 |
The equation shows the standard enthalpy of formation of CO to be -110 kJ/mol.
homologous series → homologni niz
Series of compounds which have a common general formula and in which each member differs from the next member by a constant unit, which is the methylene group (-CH2-) is called the homologous series. Members of a homologous series are called homolog.
An example of the homologous series with some of their homologs are given below. Straight chain alkanes having general formula CnH2n+2
| Structure | Name |
|---|---|
| CH4 | methane |
| CH3-CH3 | ethane |
| CH3-CH2-CH3 | propane |
| CH3-CH2CH2CH3 | butane |
| CH3-(CH2)3-CH3 | pentane |
| CH3-(CH2)4-CH3 | hexane |
| CH3-(CH2)5-CH3 | heptane |
| CH3-(CH2)6-CH3 | octane |
| CH3-(CH2)7-CH3 | nonane |
| CH3-(CH2)8-CH3 | decane |
hydrogen bond → vodikova veza
Hydrogen is a bond formed by a hydrogen atom to an electronegative atom, and is denoted by dashed lines H-X---H-B. A hydrogen atom covalently bound to an oxygen (electronegative atom) has a significant positive charge and can form a weak bond to another electronegative atom.
Citing this page:
Generalic, Eni. "Hera chan tvb." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table