ball mill → kuglični mlin
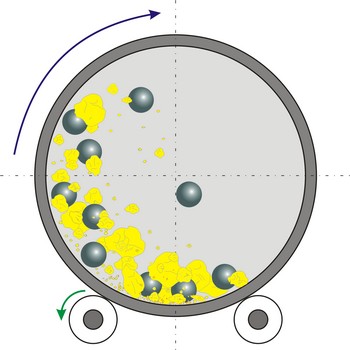
Ball mill is a grinder for reducing hard materials to powder. The grinding is carried out by the pounding and rolling of a charge of steel or ceramic balls carried within the cylinder. The cylinder rotates at a relatively slow speed, allowing the balls to cascade through the mill base, thus grinding or dispersing the materials.
Type of ball mills, centrifugal and planetary mills, are devices used to rapidly grind materials to colloidal fineness (approximately 1 μm and below) by developing high grinding energy via centrifugal and/or planetary action.
ultracentrifuge → ultracentrifuga
Ultracentrifuge is a high-speed centrifuge used in the separation of colloidal or submicroscopic particles. The ultracentrifuge can generate forces thousands or millions of times stronger than the force of gravity.
balance → vaga
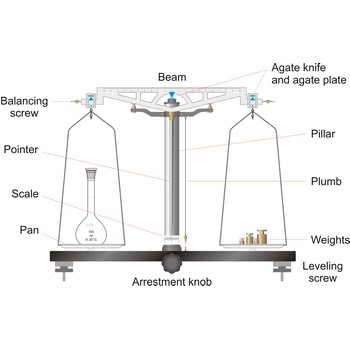
Balance is an instrument to measure the mass (or weight) of a body. Balance beam type scales are the oldest type and measure weight using a fulcrum or pivot and a lever with the unknown weight placed on one end of the lever, and a counterweight applied to the other end. When the lever is balanced, the unknown weight and the counterweight are equal. The equal-arm balance consists of two identical pans hung from either end of a centrally suspended beam. The unequal-arm balance is made with one arm of the balance much longer than the other.
More modern substitution balances use the substitution principle. In this calibrated weights are removed from the single lever arm to bring the single pan suspended from it into equilibrium with a fixed counter weight. The substitution balance is more accurate than the two-pan device and enables weighing to be carried out more rapidly.
Electromagnetic force restoration balances also use a lever system but a magnetic field is used to generate the force on the opposite end of the lever and balance out the unknown mass. The current used to drive the magnetic coil is proportional to the mass of the object placed on the platform.
benzene → benzen
Benzene is a colourless liquid hydrocarbon, C6H6, b.p. 80 °C. It is now made from petroleum by catalytic reforming (formerly obtained from coal tar). Benzene is the archetypal aromatic compound. It has an unsaturated molecule, yet will not readily undergo addition reactions. On the other hand, it does undergo substitution reactions in which hydrogen atoms are replaced by other atoms or groups.
In 1865, Friedrich August Kekulé purposed the benzene molecule structure as a hexagonal ring which consists of six carbon atoms with alternate carbon-carbon single and carbon-carbon double bond. But such a structure should be highly reactive, and so didn't account for the unreactive nature of benzene. We now know that the best representation for the structure of benzene is indeed, hexagonal, with each C-C bond distance being identical and intermediate between those for a single and double bond. The π-orbitals from each neighbouring carbon atom overlap to form a delocalised molecular orbital which extends around the ring, giving added stability and with it, decreased reactivity. That is the reason the structural formula of benzene represents as a hexagon with a circle in the center which represents the delocalized electrons.
bidentate ligand → bidentatni ligand
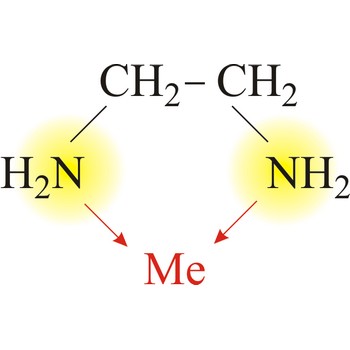
Bidentate ligand is a ligand that has two "teeth" or atoms that coordinate directly to the central atom in a complex. An example of a bidentate ligand is ethylenediamine. A single molecule of ethylenediamine can form two bonds to a metal ion. The bonds form between the metal ion and the nitrogen atoms of ethylenediamine.
chelate → kelat
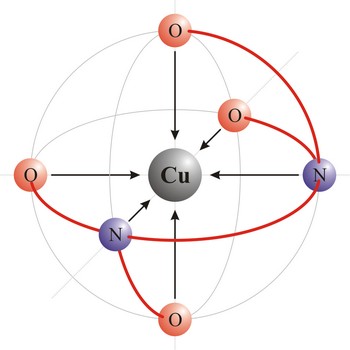
Chelate is a compound characterized by the presence of bonds from two or more bonding sites within the same ligand to a central metal atom. For example, copper complexes with EDTA to form a chelate. Chelate complexes are more stable than complexes with the corresponding monodentate ligands.
close-packed structure → gusta slagalina
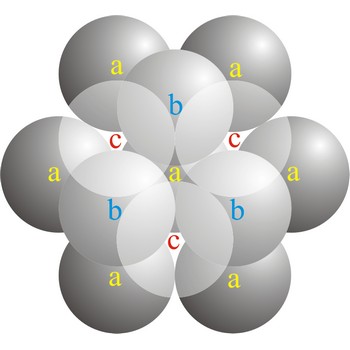
Close packing is the packing of spheres so as to occupy the minimum amount of space. The name close packed refers to the packing efficiency of 74.05 %. There are two types of close packing: hexagonal and cubic. One layer, with atoms centered on sites labeled a. Two layers, with the atoms of the second layer centered on sites labeled b. The third layer can be placed on the sites labeled c (giving cubic close-packing) or over those marked a (giving hexagonal close-packing).
cubic close-packed structure → kubična gusta slagalina
In a cubic close-packed (ccp) arrangement of atoms, the unit cell consists of four layers of atoms. The top and bottom layers (a) contain six atoms at the corners of a hexagon and one atom at the center of each hexagon. The atoms in the second layer (b) fit into depressions in the first layer. The atoms in the third layer (c) occupy a different set of depressions than those in the first. The cubic close packed structure can be made by piling layers in the a-b-c-a-b-c-a-b-c... sequence.
crystal system → kristalni sustav
Crystal system is a method of classifying crystalline substances on the basis of their unit cell. There are seven unique crystal systems. The simplest and most symmetric, the cubic (or isometric) system, has the symmetry of a cube. The other six systems, in order of decreasing symmetry, are hexagonal, tetragonal, rhombohedral (also known as trigonal), orthorhombic, monoclinic and triclinic.
|
Crystal system
|
Unit-cell
|
Conditions on unit-cell edges and angles |
|
cubic |
 |
a=b=c α=β=γ=90° |
|
hexagonal |
 |
a≠c α=γ=90° β=120° |
|
tetragonal |
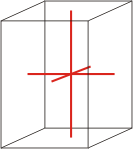 |
a=b≠c α=β=γ=90° |
|
rhombohedral |
 |
a=b=c α=β=γ≠90° |
|
orthorhombic |
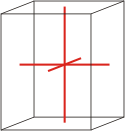 |
a≠b≠c α=β=γ=90° |
|
monoclinic |
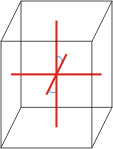 |
a≠b≠c α=γ=90°≠β |
|
triclinic |
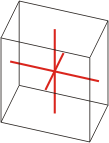 |
a≠b≠c α≠β≠γ≠90° |
Daniell cell → Daniellov članak
In 1836 the British chemist John Frederic Daniell (1790-1845) proposed an improved electric cell that supplied an even current during continuous operation. Daniell cell consisted of a glass jar containing copper and zinc electrodes, each immersed in their respective acidic sulphate solutions. The two solutions were separated by a porous clay cylinder separator. It was a galvanic cell in which the spontaneous electrodissolution of zinc and electroplating of copper provided the electrical current.
Zn(s) |
→ | Zn2+ + 2e- |
+0.763 V |
Cu2+ + 2e- |
→ | Cu(s) |
+0.337 V |
Zn(s) + Cu2+ |
→← | Zn2+ + Cu(s) |
+1.100 V |
Citing this page:
Generalic, Eni. "Centi." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table