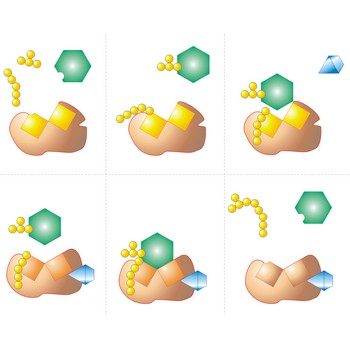
fermentation → fermentacija
Fermentation is a class of biochemical reactions that break down complex organic molecules (such as carbohydrates) into simpler materials (such as ethanol, carbon dioxide, and water). Fermentation reactions are catalyzed by enzymes.
catalyst → katalizator
Catalyst is a substance that increases the rate of a chemical reaction without itself undergoing any permanent chemical change. Catalysts that have the same phase as the reactants are homogenous catalysts (e.g. enzymes in biochemical reactions). Those that have a different phase are heterogeneous catalyst (e.g. metals or oxides used in gas reactions).
The catalyst provides an alternative pathway by which the reaction can proceed, in which the activation energy is lower. In thus increases the rate at which the reaction comes to an equilibrium, although it does not alter the position of the equilibrium.
disaccharide → disaharid
Disaccharides are compounds in which two monosaccharides are joined by a glycosidic bond. A glycosidic bond to the anomeric carbon can be either α or β. For example, maltose, the disaccharide obtained by enzyme-catalyzed hydrolysis of starch, consists of two D-glucopyranose units joined by a 1,4’-α-glycoside bond. The "prime" superscript indicates that C-4 is not in the same ring as C-1. Unlike the other disaccharides, sucrose is not a reducing sugar and does not exhibit mutarotation because the glycosidic bond is between the anomeric carbon of glucose and the anomeric carbon of fructose.
honey → med
Honey is a sweet, amber colored, viscous fluid produced by honeybees from the nectar of flowers. It is composed primarily of fructose (about 40 %), glucose (about 35 %), and water (up to 20 %). In addition, honey contains sucrose, maltose, trisaccharides, and small amounts of minerals, vitamins, and enzymes.
invertase → invertaza
Invertase (sucrase, saccharase, beta-fructofuranosidase) is an enzyme present in yeast and in the intestinal juice of animals that catalyze the hydrolysis of table sugar (sucrose, saccharose) to the simple sugars, glucose and fructose. This equimolar mixture of glucose and fructose is called invert sugar.
glutamic acid → glutaminska kiselina
Glutamic acid is an electrically charged amino acids. It is one of the two amino acids that contain a carboxylic acid group in its side chains. These acids play important roles as general acids in enzyme active centers, as well as in maintaining the solubility and ionic character of proteins. Glutamic acid is commonly referred to as glutamate, because its carboxylic acid side chain will be deprotonated and thus negatively charged in its anionic form at physiological pH. Glutamic acid is referred to as a non-essential amino acid because a healthy human can synthesize all the glutamic acid needed for normal body function from other amino acids.
- Abbreviations: Glu, E
- IUPAC name: 2-aminopentanedioic acid
- Molecular formula: C5H9NO4
- Molecular weight: 147.13 g/mol
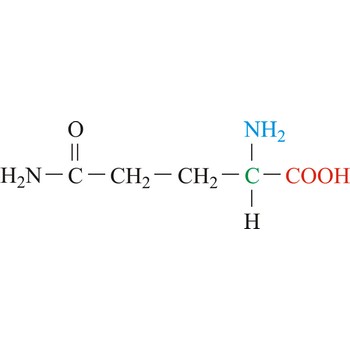
glutamine → glutamin
Glutamine is neutral amino acids with polar side chains. It serves as an important carrier of ammonia and contributes it to the formation of urea and purines. Glutamine is not recognized as an essential amino acid but may become conditionally essential in certain situations, including intensive athletic training or certain gastrointestinal disorders. It is synthesized by the enzyme glutamine synthetase from glutamate and ammonia.
- Abbreviations: Gln, Q
- IUPAC name: 2,5-diamino-5-oxopentanoic acid
- Molecular formula: C5H10N2O3
- Molecular weight: 146.14 g/mol
Citing this page:
Generalic, Eni. "Enzim." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table