limestone → vapnenac
Limestone is a sedimentary rock composed primarily of calcium carbonate in the form of the mineral calcite.. Some 10 % to 15 % of all sedimentary rocks are limestones. Limestone is usually organic, but it may also be inorganic. Calcium carbonate may have been directly precipitated from the sea-water or by the lithification of coral reefs, marine organism shells, or marine organism skeletons.
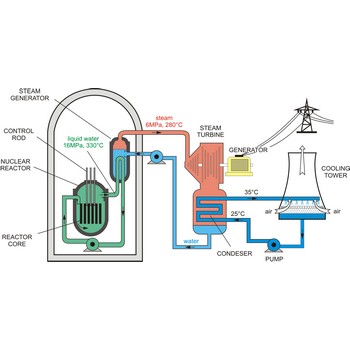
nuclear reactor → nuklearni reaktor
Nuclear reactor is an assembly of fissionable material (uranium-235 or plutonium-239) designed to produce a sustained and controllable chain reaction for the generation of electric power.
The essential components of a nuclear reactor are:
- The core, metal rods containing enough fissionable material to maintain a chain reaction at the necessary power level (as much as 50 t of uranium may be required).
- A source of neutrons to initiate the reaction (such as a mixture of polonium and beryllium)
- A moderator to reduce the energy of fast neutrons for more efficient fission (material such as graphite, beryllium, heavy water, and light water are used)
- A coolant to remove the fission-generated heat (water, sodium, helium, and nitrogen may be used)
- A control system such as rods of boron or cadmium that have high capture cross sections (to absorb neutrons)
- Adequate shielding, remote-control equipment, and appropriate instrumentation are essential for personnel safety and efficient operation.
Ostwald’s viscometer → Ostwaldov viskozimetar
Ostwald viscometer, also known as U-tube viscometer or capillary viscometer is a device used to measure the viscosity of the liquid with a known density. The method of determining viscosity with this instrument consists of measuring the time for a known volume of the liquid (the volume contained between the marks A and B) to flow through the capillary under the influence of gravity. Ostwald viscometers named after the German chemist Wilhelm Ostwald (1853-1932).
The instrument must first be calibrated with materials of known viscosity such as pure (deionized) water. Knowing the value of viscosity of one liquid, one can calculate the viscosity of other liquid.
where η1 and η2 are viscosity coefficients of the liquid and water, and ρ1 and ρ2 are the densities of liquid and water, respectively.
photomultiplier → fotomultiplikator
Photomultiplier (photomultiplier tube or PMT) is a very versatile and sensitive detector of radiant energy in the ultraviolet, visible, and near infrared regions of the electromagnetic spectrum. A typical photomultiplier tube consists of a photoemissive cathode (photocathode) followed by focusing electrodes, an electron multiplier (dynode) and an electron collector (anode) in a vacuum tube.
pipette → pipeta
Pipettes are glass tubes which are tapers towards at both ends into narrow opened tubes. According to their design two types of pipettes can be distinguished:
Volumetric pipettes
Volumetric pipettes (transfer or belly pipette) are used in volumetric analysis, when there is a need for taking exact smaller volume of a sample solution or reagent. The upper tube of volumetric pipette has a ringlike marking (mark) which marks its calibrated volume. Pipettes calibrated to deliver (TD or Ex) the indicated volume. By sucking in (with mouth, propipette or a water pump) the liquid is pulled in a little bit above the mark and the opening of the pipet is closed with a forefingertip. Outer wall of pipet is wiped and, with a slight forefinger loosening, the liquid is released until it reaches the mark. Mark must figure as a tangent on a lower edge of the liquid meniscus. A pipette is emptied out by lifting the forefinger off and letting the liquid flow out of the pipette freely. After another 15 s and the tip of the pipette is pulled onto the inner wall of the vessel. It is absolutely forbidden to blow out the contents of the pipette
Graduated pipettes
Graduated pipettes (Mohr pipette) have a scale divided into units of one and of 1/10th of a millilitre. Because of their wide necks it is less accurate than the volumetric pipette. They are used when taking volume of solutions in which accuracy does not have to be very high. They are filled in the same way as volumetric ones and liquid can be gradually released.
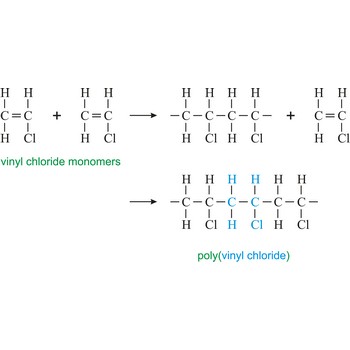
polyvinyl chloride → polivinil klorid
Poly(vinyl chloride) or the PVC is hard and resistant homopolymer produced by the polymerization of the gas vinyl chloride [CH2CHCl]. The pure polymer is hard, brittle and difficult to process, but it becomes flexible when plasticizers are added. After mixing with plasticizers, stabilizers, and pigments, the resin may be fabricated by techniques such as calendering, molding, or extrusion into flexible articles such as raincoats, shower curtains, and packaging films. The resin is not plasticized for use in making rigid products such as water pipe, plumbing fittings, and phonograph records.
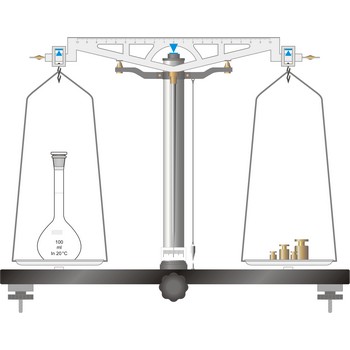
precision balance → tehnička vaga
Precision balances typically display results from three to one decimal places (0.001 g up to 0.1 g). The readability precision balances are reduced when compared to analytical balances but, precision balances accommodate higher capacities (up to several kilograms). In its traditional form, it consists of a pivoted horizontal lever of equal length arms, called the beam, with a weighing pan, also called scale, suspended from each arm.
In electronic top pan, or toploader balances, mass is determined not by mechanical deflection but by electronically controlled compensation of an electric force. The signal generated enables the mass to be read from a digital display. The mass of the empty container can be stored in the balance’s computer memory and automatically deducted from the mass of the container plus its contents.
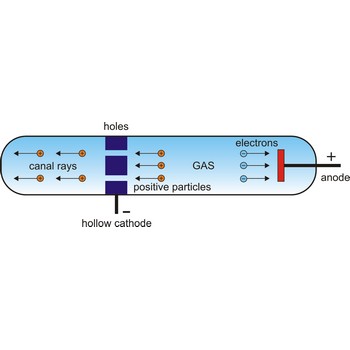
proton → proton
Proton is a stable elementary particle of unit positive charge and spin 1/2. Protons and neutrons, which are collectively called nucleons, are the constituents of the nucleus.
In 1886, German physicist Eugene Goldstein (1850-1930) discovered positive particles by using a modified Crookes tube with holes in the cathode in an evacuated tube. When cathode rays were given off in one direction toward the anode, other rays found their way through the holes in the cathode and sped off in the opposite direction. Since these other rays traveled in the direction opposite to the negatively charged cathode rays, it seemed that they must be composed of positively charged particles. Rutherford suggested that this fundamental positive particle be called the proton.
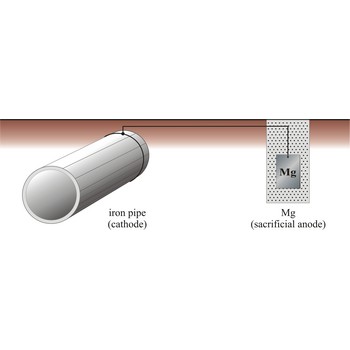
sacrificial protection → zaštita žrtvovanom elektrodom
Sacrificial protection is the protection of iron or steel against corrosion by using a more reactive metal. Pieces of zinc or magnesium alloy are attached to pump bodies and pipes. The protected metal becomes the cathode and does not corrode. The anode corrodes, thereby providing the desired sacrificial protection. These items are known as sacrificial anodes and "attract" the corrosion to them rather than the iron/steel. The sacrificial anodes must be replaced periodically as they corrode.
The iron pipe will be connected to a more reactive metal such as magnesium through cooper wires, the magnesium will donate its electrons to the iron preventing it from rusting. Iron which is oxidises will immediately be reduced back to iron.
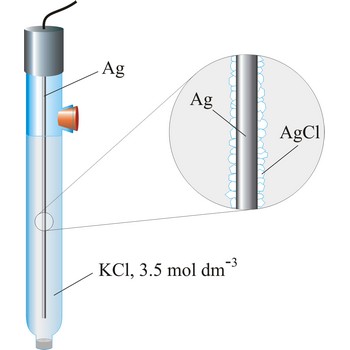
silver/silver-chloride electrode → srebro/srebrov klorid elektroda
Silver/silver-chloride electrode is by far the most common reference type used today because it is simple, inexpensive, very stable and non-toxic. It is mainly used with saturated potassium chloride electrolyte, but can be used with lower concentrations such as 3.5 mol dm-3 or 1 mol dm-3 potassium chloride. Silver/silver-chloride electrode is a referent electrode based on the following halfreaction
| Potential vs. SHE / V | ||
|---|---|---|
| t / °C | 3.5 mol dm-3 | sat. solution |
| 15 | 0.212 | 0.209 |
| 20 | 0.208 | 0.204 |
| 25 | 0.205 | 0.199 |
| 30 | 0.201 | 0.194 |
| 35 | 0.197 | 0.189 |
Citing this page:
Generalic, Eni. "Wagnerova cijev." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table