retardation factor → faktor zaostajanja
Retardation factor, RF, (in planar chromatography) is a ratio of the distance travelled by the centre of the spot to the distance simultaneously travelled by the mobile phase:
The RF value is characteristic for any given compound on the same stationary phase using the same mobile phase for development of the plates. Hence, known RF values can be compared to those of unknown substances to aid in their identifications.
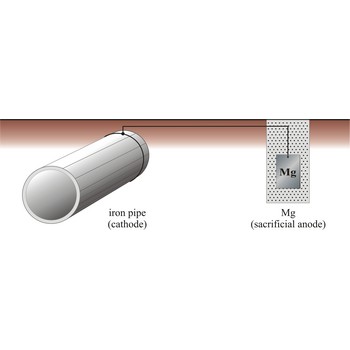
sacrificial protection → zaštita žrtvovanom elektrodom
Sacrificial protection is the protection of iron or steel against corrosion by using a more reactive metal. Pieces of zinc or magnesium alloy are attached to pump bodies and pipes. The protected metal becomes the cathode and does not corrode. The anode corrodes, thereby providing the desired sacrificial protection. These items are known as sacrificial anodes and "attract" the corrosion to them rather than the iron/steel. The sacrificial anodes must be replaced periodically as they corrode.
The iron pipe will be connected to a more reactive metal such as magnesium through cooper wires, the magnesium will donate its electrons to the iron preventing it from rusting. Iron which is oxidises will immediately be reduced back to iron.
solar cell → sunčeva ćelija
Solar cell, or photovoltaic cell, is a device that captures sunlight and transforms it directly to electricity. All solar cells make use of photovoltaic effect, so often they are called photovoltaic cells. Almost all solar cells are built from solid-state semiconducting materials, and in the vast majority of these the semiconductor is silicon.
The photovoltaic effect involves the generation of mobile charge carriers-electrons and positively charged holes-by the absorption of a photon of light. This pair of charge carriers is produced when an electron in the highest filled electronic band of a semiconductor (the valence band) absorbs a photon of sufficient energy to promote it into the empty energy band (the conduction band). The excitation process can be induced only by a photon with an energy corresponding to the width of the energy gap that separates the valence and the conduction band. The creation of an electron-hole pair can be converted into the generation of an electrical current in a semiconductor junction device, wherein a layer of semiconducting material lies back to back with a layer of either a different semiconductor or a metal. In most photovoltaic cells, the junction is p-n junction, in which p-doped and n-doped semiconductors are married together. At the interface of the two, the predominance of positively charged carriers (holes) in the p-doped material and of negatively charged carriers (electrons) in the n-doped material sets up an electric field, which falls off to either side of the junction across a space-charge region. When absorption of a photon in this region generates an electron-hole pair, these charge carriers are driven in opposite directions by the electric field, i.e. away from the interface and toward the top and bottom of the two-layer structure, where metal electrodes on these faces collect the current. The electrode on the top layer (through which light is absorbed) is divided into strips so as not to obscure the semiconducting layers below. In most widely used commercial solar cells, the p-doped and n-doped semiconductive layers are formed within a monolithic piece of crystalline silicon. Silicon is able to absorb sunlight at those wavelengths at which it is most intense-from the near-infrared region (wavelengths of around 1200 nm) to the violet (around 350 nm).
solid state → čvrsto agregatno stanje
Solid state is characterised by a constant shape and volume. Particles are placed very close to one another and have efect one on another with great attraction forces. Solid bodies do not assume the shape of the container in which they are put.
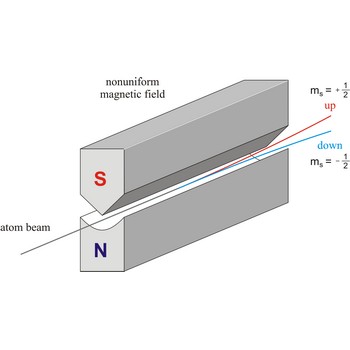
spin → spin
Spin is the intrinsic angular momentum of an elementary particle, or system of particles such as nucleus, that is also responsible for the magnetic moment; or, a particle or nucleus possessing such a spin. The spins of nuclei have characteristic fixed values. Pairs of neutrons and protons align to cancel out their spins, so that nuclei with an odd number of neutrons and/or protons will have a net non-zero rotational component characterized by a non-zero quantum nuclear spin number.
Stern-Gerlach experiment: a beam of silver atoms is split into two beams when it traverses a nonuniform magnetic field. Atoms with spin quantum number ms=+1/2 follow one trajectory, and those with ms=+1/2 follow another.
stoichiometry → stehiometrija
Stoichiometry is the relative proportions elements from compounds or in which substances react. Every chemical reaction has its characteristic proportions. For example, when methane unites with oxygen in complete combustion, 1 mol of methane requires 2 mol of oxygen.
At the same time, 1 mol of carbon dioxide and 2 mol of water are formed as reaction products.
Alternatively, 16 g of methane and 64 g of oxygen produce 44 g of carbon dioxide and 36 g of water.
The stoichiometric relationship between the products and reactants can be used to in calculations.
stratosphere → stratosfera
Stratosphere is the part of the earth’s atmosphere extending from the top of the troposphere (typically 10 km to 15 km above the surface) to about 50 km. It is characterised by an increase in temperature with increasing altitude.
superfluid helium → superfluidni helij
Superfluidity in helium-4 was discovered in 1938 by the Soviet physicist Pyotr Leonidovich Kapitsa. Helium-4 exhibits superfluidity when it is cooled below 2.18 K (-270.97 C), which is called the lambda (λ) point. At these temperatures, helium-4 exhibits the characteristics of two distinct fluids, one of which appears to flow without friction. An extensive series of experiments showed that in this state of helium, called helium II (He II), there is an apparent enormous rise in heat conductivity, at an increase rate of about three million. Another unusual property of He II is its mobile, rapid flow through capillaries or over the rim of its containment vessel as a thin film that exhibits no measurable viscosity and appears unaffected by the forces of gravity or evaporation and condensation.
thermosphere → termosfera
Thermosphere is the layer of the earth’s atmosphere extending from the top of the mesosphere (80 km - 90 km above the surface) to about 500 km. It is characterised by a rapid increase in temperature with increasing altitude up to about 200 km, followed by a levelling off in the 300 km - 500 km region.
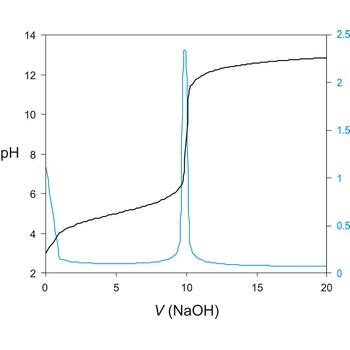
titration curve → titracijska krivulja
Titration curve is a graphic representation of the amount of a species present vs. volume of solution added during a titration. A titration curve has a characteristic sigmoid curve. The inflection point in the titration curve marks the end-point of the titration. Blue line is the first derivative of the titration curve.
Citing this page:
Generalic, Eni. "Wire gauge chart." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table