catalytic hydrogenation → katalitičko hidrogeniranje
Catalytic hydrogenation is the infusing of unsaturated or impure hydrocarbons with hydrogen gas at controlled temperatures and pressures and in the presence of a catalyst for the purpose of obtaining saturated hydrocarbons and/or removing various impurities such as sulphur and nitrogen.
coal gas → ugljeni plin
Coal gas is a gas produced by the destructive distillation of coal, and contains approximately 50 % hydrogen, 35 % methane and 8 % carbon monoxide. The by-products of the production of coal gas are coal tar and coke.
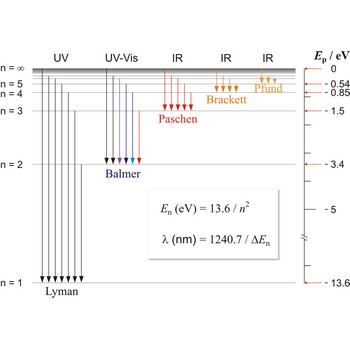
Balmer series → Balmerova serija
Balmer series, Balmer lines is a series of lines in the emission spectrum of hydrogen that involve transitions to the n=2 state from states with n>2.
benzene → benzen
Benzene is a colourless liquid hydrocarbon, C6H6, b.p. 80 °C. It is now made from petroleum by catalytic reforming (formerly obtained from coal tar). Benzene is the archetypal aromatic compound. It has an unsaturated molecule, yet will not readily undergo addition reactions. On the other hand, it does undergo substitution reactions in which hydrogen atoms are replaced by other atoms or groups.
In 1865, Friedrich August Kekulé purposed the benzene molecule structure as a hexagonal ring which consists of six carbon atoms with alternate carbon-carbon single and carbon-carbon double bond. But such a structure should be highly reactive, and so didn't account for the unreactive nature of benzene. We now know that the best representation for the structure of benzene is indeed, hexagonal, with each C-C bond distance being identical and intermediate between those for a single and double bond. The π-orbitals from each neighbouring carbon atom overlap to form a delocalised molecular orbital which extends around the ring, giving added stability and with it, decreased reactivity. That is the reason the structural formula of benzene represents as a hexagon with a circle in the center which represents the delocalized electrons.
Biot-Savart law → Biot-Savartov zakon
The magnetic field B due to a current-carrying conductor can be determined by Biot-Savart law. The contribution to magnetic field set up at distance r by the current element IdL is given by expression:
where μ0 is permeability constant. It plays a role in magnetic problems equivalent to the role of permittivity constant μ0 in electrostatics problems. In order to obtain B, contributions of all current elements have to be integrated. In case of a long straight conductor, carrying current I, Biot-Savart law gives:
SI unit for magnetic field B is tesla (T).
Permaeability constant μ0 has value 4π×10-7 T m A-1.
bismuth → bizmut
Bismuth was discovered by Claude Geoffroy (France) in 1753. The origin of the name comes from the German words Weisse Masse meaning white mass; now spelled wismut and bisemutum. It is hard, brittle, steel-grey metal with a pink tint. Stable in oxygen and water. Dissolves in concentrated nitric acid. Bismuth can be found free in nature and in minerals like bismuthine (Bi2S3) and in bismuth ochre (Bi2O3) Main use is in pharmaceuticals and low melting point alloys used as fuses.
borane → borani
Borane is any of the group of compounds of boron and hydrogen (B2H6, B4H10, B5H9, B5H11...), many of which can be prepared by action of acid on magnesium boride (Mg3B2). Boranes are a remarkable group of compounds in that their structures cannot be described using the conventional two-electron covalent bond model.
brass → mjed
Brasses are alloys of copper and zinc (generally 5 % to 40 %). Brass has been known to man since prehistoric times, long before zinc itself was discovered. It was produced by melting copper together with calamine, a zinc ore. Its ductility reaches a maximum with about 30 % zinc and its tensile strength with 45 % although this property varies greatly with the mechanical and heat treatment of the alloy. Typical applications included gears, plumbing ware fittings, adapters, valves and screw machine products. The French horn is a valved brass wind instrument.
Brass may contain small amounts of other alloying elements, such as aluminum, lead, tin, or nickel. Lead can be added as an alloying element resulting in a brass that can be rapidly machined and produces minimal tool wear. Additions of aluminium, iron and manganese to brass improve strength, whilst silicon additions improve wear resistance. Brass containing tin (< 2 % ) is less liable to corrosion in seawater; it is sometimes called naval brass and is used in naval construction.

covalentity → kovalentnost
Covalentity is a maximum number of covalent bond that one atom can make, it is equal to the number of hydrogen atoms that are combined with other atoms. It is usually constant.
decomposing → raščinjavanje
Decomposing in analytical chemistry means that a certain substance is converted, by melting it with a suitable melting medium (sodium carbonate, sodium hydroxide, sodium peroxide, ...) in the kind of compound which will afterwards that dissolve in water, acid or base very easily.
Citing this page:
Generalic, Eni. "Vodik." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table