galvanic celll → galvanski članak
Galvanic cell (voltaic cell) is a simple device with which chemical energy is converted into electrical energy. Galvanic cells consist of two separate compartments called half cells containing electrolyte solutions and electrodes that can be connected in a circuit. Two dissimilar metals (e.g., copper and zinc) are immersed in an electrolyte. If the metals are connected by an external circuit, one metal is reduced (i.e., gains electrons) while the other metal is oxidized (i.e., loses electrons).
In the example above, copper is reduced and zinc is oxidized. The difference in the oxidation potentials of the two metals provides the electric power of the cell.
A voltaic cell can be diagrammed using some simple symbols. In the diagram the electrodes are on the outer side of the diagram and a vertical line (|) is used to separate the electrode from the electrolyte solution found in the compartment. A double vertical line (||) is used to separate the cell compartments and is symbolic of the salt bridge. Usually in a diagram the species oxidized is written to the left of the double slash. Here is an example of the Daniell cell:
The names refer to the 18th-century Italian scientists Alessandro Volta (1745-1827) and Luigi Galvani (1737-1798).
gas liquefying → ukapljivanje plinova
In order to achieve transition of a gas into liquid state it is necessary to lower its temperature, or decrease its volume, or increase its pressure. Above the critical temperature it is impossible to liquefy a gas. When liquefying a gas by Linde’s procedure, dampening or Joule-Thomson’s effect is used. First, the compressed air from the compressor is cooled with cooling water, the cooled air expands at a lower pressure in the dampening valve at which it cooled. The cooled air now returns to the compressor, cooling down the expanding air. By repeating this process the air is cooled enough to transit to the liquid state.
Gratzel solar cell → Gratzelova sunčeva ćelija
Grätzel solar cell is photoelectrochemical cell, developed by Michael Grätzel and collaborators, simulates some characteristics of the natural solar cell, which enables photosynthesis take place. In natural solar cell the chlorophyll molecules absorb light (most strongly in the red and blue parts of the spectrum, leaving the green light to be reflected). The absorbed energy is sufficient to knock an electron from the excited chlorophyll. In the further transport of electron, other molecules are involved, which take the electron away from chlorophyll. In Grätzel cell, the tasks of charge-carrier generation and transport are also assigned to different species.
His device consists of an array of nanometre-sized crystallites of the semiconductor titanium dioxide, welded together and coated with light-sensitive molecules that can transfer electrons to the semiconductor particles when they absorb photons. So, light-sensitive molecules play a role equivalent to chlorophyll in photosynthesis. In Grätzel cell, the light-sensitive molecule is a ruthenium ion bound to organic bipyridine molecules, which absorb light strongly in the visible range; titanium dioxide nanocrystals carry the received photoexcited electrons away from electron donors. On the other hand, a donor molecule must get back an electron, so that it can absorb another photon. So, this assembly is immersed in a liquid electrolyte containing molecular species (dissolved iodine molecules) that can pick up an electron from an electrode immersed in the solution and ferry it to the donor molecule. These cells can convert sunlight with efficiency of 10 % in direct sunlight and they are even more efficient in diffuse daylight.
hassium → hassij
Hassium was discovered by Peter Armbruster, Gottfried Münzenber and their co-workers at the Heavy Ion Research Laboratory (Gesellschaft für Schwerionenforschung, GSI) in Darmstadt, Germany in 1984. The origin of the name is the Latin word Hassias meaning Hess, the German state. It is synthetic radioactive metal. Hassium was produced bythe bombardment of lead-208 with iron-58.
hydrogen bond → vodikova veza
Hydrogen is a bond formed by a hydrogen atom to an electronegative atom, and is denoted by dashed lines H-X---H-B. A hydrogen atom covalently bound to an oxygen (electronegative atom) has a significant positive charge and can form a weak bond to another electronegative atom.
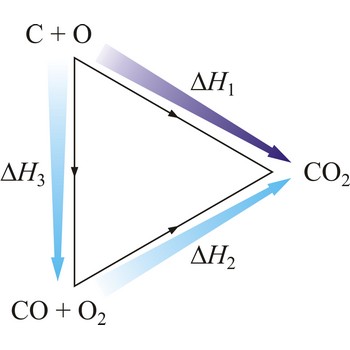
Hesse’s law → Hessov zakon
Hesse’s law says that reaction heat of some chemical change does not depend on the way in which the reaction is conducted, but only on starting and ending system state. Hesse’s law is also known as the law of constant heat summation. Hesse’s law is also known as the law of constant heat summation. The law was first put forward in 1840 by the Swiss-born Russian chemist Germain Henri Hess (1802-1850).
Hesse’s law can be used to obtain thermodynamic data that cannot be measured directly. For example, it is very difficult to control the oxidation of graphite to give pure CO. However, enthalpy for the oxidation of graphite to CO2 can easily be measured. So can the enthalpy of oxidation of CO to CO2. The application of Hess’s law enables us to estimate the enthalpy of formation of CO.
| C(s) + O2(g) →← CO2(g) | ΔrH1 = -393 kJ mol-1 |
| CO(g) + 1/2O2(g) →← CO2(g) | ΔrH2 = -283 kJ mol-1 |
| C(s) + 1/2O2(g) →← CO(g) | ΔrH3 = -110 kJ mol-1 |
The equation shows the standard enthalpy of formation of CO to be -110 kJ/mol.
hypsometric curve → hipsometrijska krivulja
Hypsometric curve (or hypsographic curve) shows the distribution of height of a given area (on land) and depth (at sea). The term originates from the Greek word hypsos meaning height. The part of the curve that reflects the cross section of the ocean bottom is called the bathygraphic curve.
Horizontal dashed lines indicate average height of the continents at 840 meters above sea level, and average depth of the oceans at 3 682.2 meters below sea level. If all the land above sea level (green) was moved into the sea (blue), the oceans would still be 3 km deep.
indium → indij
Indium was discovered by Ferdinand Reich and Hieronymus Theodor Richter (Germany) in 1863. Named after the indicum (colour indigo), the colour it shows in a spectroscope. It is rare, very soft, silver-white metal. Stable in air and water. Dissolves in acids. Metal can ignite and burn. Indium is found in certain zinc ores. Used to coat high speed bearings and as an alloy that lowers the melting point of other metals. Relatively small amounts are used in dental items and in electronic semiconductors.
Kjeldahl flask → Kjeldahlova tikvica
Kjeldahl flask is a round bottom flask with a long wide neck that is used in the determination of nitrogen by Kjeldahl’s method. The method was developed by the Danish chemist Johan Kjeldahl (1849-1900).
Kjeldahl’s method → Kjeldahlov postupak
Kjeldahl’s method is an analytical method for determination of nitrogen in certain organic compounds. The method was developed by the Danish chemist Johan Kjeldahl (1849-1900).
It involves addition of a small amount of anhydrous potassium sulphate to the test compound, followed by heating the mixture with concentrated sulphuric acid, often with a catalyst such as copper sulphate. As a result ammonia is formed. After alkalyzing the mixture with sodium hydroxyde, the ammonia is separated by distillation, collected in standard acid, and the nitrogen determined by back-titration.
- Kjeldahl flask for decomposition (500 ml – macro or 100 ml - micro)
- funnel for alkaline solution
- Wagner tube (drop catcher)
- condenser
- absorption flask with known volume of standard acid
Citing this page:
Generalic, Eni. "Truyện hoạt hình hiếp dâm mutsuzy kimetsu no yaiba." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table