X-ray diffraction pattern → rendgenski difrakcijski uzorci
X-ray diffraction pattern is an interference pattern created by x-rays as they pass through a solid material. Studying X-ray diffraction patterns gives detailed information on the three-dimensional structure of crystals, surfaces, and atoms.
Frasch proces → Fraschov postupak
Frasch proces is a method of obtaining sulphur from underground deposits using a tube consisting of three concentric pipes. Superheated steam is passed down the outer pipe to melt the sulphur, which is forced up through the middle pipe by compressed air fed through the inner tube. The steam in the outer casing keeps the sulphur molten in the pipe. It was named after the German-born American chemist Herman Frasch (1851-1914).
gamma radiation → gama-zračenje
Gamma radiation is electromagnetic radiation of extremely short wavelength. Gamma radiation ranges in energy from about 10-15 J to 10-10 J (10 keV to 10 MeV) (wavelength less than about 1 pm). Gamma rays are emitted by excited atomic nuclei during the process of passing to a lower excitation state.
Gamma rays are extremely penetrating and are absorbed by dense materials like lead and uranium. Exposure to gamma radiation may be lethal.
Geiger counter → Geigerov brojač
Geiger counter (Geiger-Muller counter) is a device used to detect and measure ionising radiation. It consists of a tube containing a low-pressure gas (usually argon or neon with methane) and a cylindrical hollow cathode through the centre of which runs a fine-wire anode. A potential difference of about 1 000 V is maintained between the electrodes. An ionising particle or photon passing through a window into the tube will cause an ion to be produced and the high potential will accelerate it towards its appropriate electrode, causing an avalanche of further ionisations by collision. The consequent current pulses can be counted in electronic circuits or simply amplified to work a small loudspeaker in the instrument. It was first devised in 1908 by the German physicist Hans Geiger (1882-1945). Geiger and W. Muller produced an improved design in 1928.
global hectare → globalni hektar
Global hectares (gha) are hectares with world-average productivity for all productive land and water areas in a given year. Because different land types have different productivity, a global hectare of, for example, cropland, would occupy a smaller physical area than the much less biologically productive pasture land, as more pasture would be needed to provide the same biocapacity as one hectare of cropland ("ordinary" hectare is an area equal to a square that is 100 meters on each side, so a hectare has 10 000 m2). Global hectare is unit for measuring our demands on the Earth (ecological footprint) and the ability of the Earth to supply our demands (biocapacity).
global warming → globalno zatopljenje
Global warming or greenhouse effect is an effect occurring in the atmosphere because of the presence of certain gases (greenhouse gases) that absorb infrared radiation. Light and ultraviolet radiation from the sun is able to penetrate the atmosphere and warm the Earth’s surface. This energy is re-radiated as infrared radiation which because of its longer wavelength, is absorbed by such substances as carbon dioxide. The overall effect is that the average temperature of the Earth and its atmosphere is increasing (so-called global Warming). The effect is similar to that occurring in a greenhouse, where light and long-wavelength ultraviolet radiation can pass through the glass into greenhouse but the infrared radiation is absorbed by the glass and part of it is re-radiated into the greenhouse.
The greenhouse effect is seen as a major environmental hazard. Average increases in temperature could change weather patterns and agricultural output. It might also lead to melting of the polar ice caps and a corresponding rise in sea level. Carbon dioxide, from fossil-fuel power stations and car exhausts, is the main greenhouse gas. Other contributory pollutants are nitrogen oxides, ozone, methane, and chloroflourocarbons.
half-life of a reactant → poluživot reaktanta
For a given reaction the half-life, t1/2, of a reactant is the time required for its concentration to reach a value that is the arithmetic mean of its initial and final (equilibrium) value.
Half-life is constant for first-order reactions.
Half-life is not constant for second-order reactions but rather it varies with initial concentration and k.
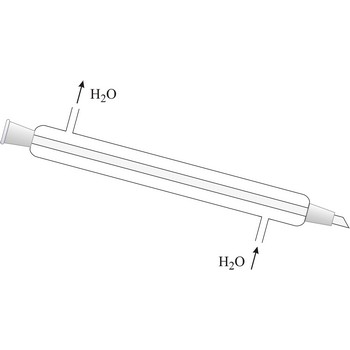
Liebig condenser → Liebigovo hladilo
Liebig condenser is used for condensing of vapours that pass trough the centre tube. It is cooled with water that passes in the outer tube (shell around the centre tube) in the opposite direction than the one of hot vapour. Though named after the German chemist Justus von Liebig (1803-1873), he cannot be given credit for having invented it because it had already been in use for some time before him.
lithium → litij
Lithium was discovered by Johan August Arfvedson (Sweden) in 1817. The origin of the name comes from the Greek word lithos meaning stone, apparently because it was discovered from a mineral source whereas the other two elements, sodium and potassium, were discovered from plant sources. It is soft silvery-white metal. Lightest of metals. Reacts slowly with water and oxygen. Flammable. Can ignite in air. Reacts with water to give off a flammable gas. Lithium is obtained by passing electric charge through melted lithium chloride and from the silicate mineral called spodumene [LiAl(Si2O6)]. Used in batteries. Also for certain kinds of glass and ceramics. Some is used in lubricants.
optically active matter → optički aktivna tvar
Optically active matter, by polarized light passing through it, turns the flat of polarized light leftwards or rightwards.
Citing this page:
Generalic, Eni. "To pass final judgement中文." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table