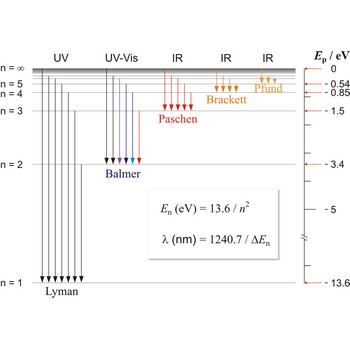
Paschen series → Paschenova serija
Paschen series are the series of lines in the spectrum of the hydrogen atom which corresponds to transitions between the state with principal quantum number n = 3 and successive higher states.
electrochemical cell → elektrokemijski članak
Electrochemical cell is a device that converts chemical energy into electrical energy or vice versa when a chemical reaction is occurring in the cell. It consist of two electronically conducting phases (e.g., solid or liquid metals, semiconductors, etc) connected by an ionically conducting phase (e.g. aqueous or non-aqueous solution, molten salt, ionically conducting solid). As an electric current passes, it must change from electronic current to ionic current and back to electronic current. These changes of conduction mode are always accompanied by oxidation/reduction reactions.
An essential feature of the electrochemical cell is that the simultaneously occurring oxidation-reduction reactions are spatially separated. E.g., in a spontaneous chemical reaction during the oxidation of hydrogen by oxygen to water, electrons are passed directly from the hydrogen to the oxygen.
In contrast, in the spontaneous electrochemical reaction in a galvanic cell the hydrogen is oxidised at the anode by transferring electrons to the anode and the oxygen is reduced at the cathode by accepting electrons from the cathode. The ions produced in the electrode reactions, in this case positive hydrogen ions and the negative hydroxyl (OH-) ions, will recombine in the solution to form the final product of the reaction: water. During this process the electrons are conducted from the anode to the cathode through an outside electric circuit where the electric current can drive a motor, light a light bulb, etc. The reaction can also be reversed: water can be decomposed into hydrogen and oxygen by the application of electrical power in an electrolytic cell.
absorption → apsorpcija
Absorption is a phenomenon that occurs when matter crosses from one phase to another passing through the border surface and in the other phase more or less monotonously distributes itself in a concentration higher than the one within the first phase.
acceleration → akceleracija
If a point-like object undergoes a change in velocity Δv=vf-vi in time Δt=tf-ti (indexes i and f stand for initial and final instant as well as for initial and final velocity) its average acceleration, a is defined as
The instantaneous acceleration, a, is obtained from the average acceleration by shrinking the time interval Δt towards zero. The average acceleration approaches a limiting value, which is the acceleration of a given instant:
Acceleration is a vector quantity. SI unit for acceleration is m s-2.
accumulator → akumulator
Accumulator (secondary cell, storage battery) is a type of voltaic cell or battery that can be recharged by passing current through it from an external D.C. supply. The charging current reverses the chemical reactions in the cell. The common types are the lead-acid accumulator and the nickel-cadmium cell.
air stripping → zračni striping
Air stripping is a process for the removal of volatile organic contaminants from groundwater. The groundwater flows downward inside a tower filled with materials (the packing) over a large surface area. Air is introduced at the bottom of the tower and is forced upward past the falling water. The volatiles evaporate from the water and are collected in air filters or released to the atmosphere.
alpha rays → alfa-zrake
Alpha rays are a stream of alpha particles. Alpha rays rapidly dissipate their energy as they pass through materials. They were discovered by the British physicist Ernest Rutherford (1871-1937), a New Zealander by birth, in 1903.
amperometry → amperometrija
Amperometry is determining the concentration of a material in a sample by measuring electric current passing through a cell containing the solution.
Citing this page:
Generalic, Eni. "To pass final judgement中文." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table