practical salinity → praktični salinitet
Practical salinity SP is defined on the Practical Salinity Scale of 1978 (PSS-78) in terms of the conductivity ratio K15 which is the electrical conductivity of the sample at temperature t68 = 15 °C and pressure equal to one standard atmosphere, divided by the conductivity of a standard potassium chloride (KCl) solution at the same temperature and pressure. The mass fraction of KCl in the standard solution is 0.0324356 (32.4356 g of KCl in 1 kg of solution). When K15 = 1, the Practical Salinity P S is by definition 35. The conductivity of that reference solution is C(35,1568,0) = 42.914 mS/cm = 4.2914 S/m (Siemens per meter). Note that Practical Salinity is a unit-less quantity. Though sometimes convenient, it is technically incorrect to quote Practical Salinity in "psu". When K15 is not unity, SP and K15 are related by the PSS-78 equation
At a temperature of t68 = 15 °C, Rt is simply K15 and Practical Salinity SP can be determined from the above equation. For temperatures other than t68 = 15 °C, Practical Salinity SP is given by the following function of Rt (k = 0.0162)
salinity → salinitet
Salinity (S) is a measure of the quantity of dissolved salts in seawater. It is formally defined as the total amount of dissolved solids in seawater in parts per thousand (‰) by weight when all the carbonate has been converted to oxide, the bromide and iodide to chloride, and all organic matter is completely oxidized.
Chlorinity is the oldest of the salinity measures considered and is still a corner-stone in the study of dissolved material in seawater. Based on the principle of constant relative proportions it provides a measure of the total amount of dissolved material in seawater in terms of the concentration of halides. The relationship between chlorinity (Cl) and salinity as set forth in Knudsen’s tables is
In 1962, however, a better expression for the relationship between total dissolved salts and chlorinity was found to be
Practical Salinity (SP) was introduced as a replacement for Chlorinity. Practical Salinity is is relatively easy to measure using standard conductometers, measurements are more precise and less time consuming than measurements of Chlorinity and accurate measurements can even be made in situ. Practical salinity SP is defined on the Practical Salinity Scale of 1978 (PSS-78) in terms of the conductivity ratio K15 which is the electrical conductivity of the sample at temperature t68 = 15 °C and pressure equal to one standard atmosphere, divided by the conductivity of a standard potassium chloride (KCl) solution at the same temperature and pressure. The mass fraction of KCl in the standard solution is 0.0324356 (32.4356 g of KCl in 1 kg of solution).
Note that Practical Salinity is a unit-less quantity. Though sometimes convenient, it is technically incorrect to quote Practical Salinity in "psu". For most purposes one can assume that the psu and the ‰, are synonymous.
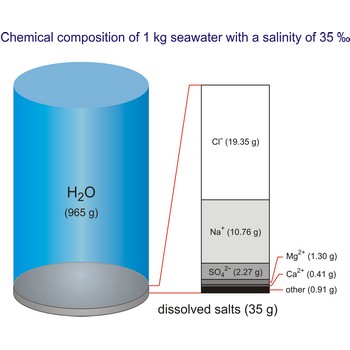
The global average salinity of ocean waters is about 35 ‰, that is, about 35 g of solid substances are dissolved in 1 kg of seawater.
seawater → more
Seawater is a complex mixture of 96.5 % water, 3.5 % salts, and smaller amounts of other substances, including dissolved inorganic and organic materials, particulates, and a few atmospheric gases. The world's oceans cover nearly 71 % (361 840 000 km2) of the Earth's surface (510 100 000 km2), with an average depth of 3 682.2 m.
The density of seawater is higher than that of fresh water because of its higher salinity. Seawater's freezing point is lower than that of pure water and its boiling point is higher. The average salinity of the ocean is 35 ‰, which means that for every kilograms of water, there are 35 g of salt. The relative abundance of the major salts in seawater are constant regardless of the ocean. Only six elements and compounds comprise about 99 % of sea salts: chlorine (Cl-), sodium (Na+), sulfur (SO42-), magnesium (Mg2+), calcium (Ca2+), and potassium (K+).
Soxhlet extractor → Soxhletov ekstraktor
Soxhlet extractor is a laboratory apparatus designed to extract substances with a low solubility in the extracting solvent. The method described by the German chemist Franz von Soxhlet (1848-1926) in 1879 is the most commonly used example of a semi-continuous method applied to extraction of lipids from foods. In the Soxhlet extractor, the sample soaks in hot solvent that is periodically siphoned off, distilled and returned to the sample. During each cycle, a portion of the non-volatile compound dissolves in the solvent. After many cycles the desired compound is concentrated in the distillation flask. The solvent in the flask is then evaporated and the mass of the remaining lipid is measured.
voltaic pile → Voltin stup
Voltaic pile was the first device that produced a continuous electric current. The first piles constructed by the Italian physicist Alessandro Volta (1745-1827) in 1800 comprised alternating silver and zinc discs separated by cardboard soaked in brine. The pile can be stacked as high as you like, and each layer will increase the voltage by a fixed amount.
Wagner tube → Wagnerova cijev
Wagner tube or drop catcher impedes the movement of distillate drops from the distillation flask to the condensing chamber.
T-S diagram → T-S dijagram
The relationship between the temperature (T) and the salinity (S) of a seawater can be illustrated graphically on a T-S diagram, which is a simple, but powerful tool used in studies of seawater density, mixing, and circulation. In a T-S diagram, temperature is plotted along the vertical axis in degrees Celsius and salinity is measured along the horizontal axis in PSU (Practical Salinity Units). Seawater density is illustrated in the diagram by curved lines of constant density (isopycnals). Water tends to move horizontally throughout the deep ocean, moving along lines of equal density.
Citing this page:
Generalic, Eni. "Slana komora." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table