Results 1–7 of 7 for reaktivnost
reactivity → reaktivnost
Reactivity is the tendency of a compound to chemically react with other substances or itself, resulting in the release of energy.
alkali metal → alkalijski metal
Alkali metal is a term that refers to six elements: lithium (Li), sodium (Na), potassium (K), rubidium (Rb), caesium (Cs), and francium (Fr). These elements make up group 1 of the periodic table of elements. They all form singly charged positive ions, and are extremely reactive. They react violently with water, forming hydroxides and releasing hydrogen gas and heat. Caesium and francium are the most reactive and lithium is the least.
alkaline earth metal → zemnoalkalijski metal
Alkali earth metal is a term that refers to six elements: beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra). These elements make up group 2 of the periodic table of elements. They all exhibit a single oxidation state, +2. They are all light and very reactive. Barium and radium are the most reactive and beryllium is the least.
To denote slightly soluble metal oxides chemists formerly used the term "earth". The oxides of barium, strontium, and calcium resemble alumina (Al2O3), a typical "earth", but form alkaline mixtures with water. For this reason barium, strontium, and calcium were called alkaline earth metals. This name has now been extended to include all of the elements of group 2.
benzene → benzen
Benzene is a colourless liquid hydrocarbon, C6H6, b.p. 80 °C. It is now made from petroleum by catalytic reforming (formerly obtained from coal tar). Benzene is the archetypal aromatic compound. It has an unsaturated molecule, yet will not readily undergo addition reactions. On the other hand, it does undergo substitution reactions in which hydrogen atoms are replaced by other atoms or groups.
In 1865, Friedrich August Kekulé purposed the benzene molecule structure as a hexagonal ring which consists of six carbon atoms with alternate carbon-carbon single and carbon-carbon double bond. But such a structure should be highly reactive, and so didn't account for the unreactive nature of benzene. We now know that the best representation for the structure of benzene is indeed, hexagonal, with each C-C bond distance being identical and intermediate between those for a single and double bond. The π-orbitals from each neighbouring carbon atom overlap to form a delocalised molecular orbital which extends around the ring, giving added stability and with it, decreased reactivity. That is the reason the structural formula of benzene represents as a hexagon with a circle in the center which represents the delocalized electrons.
reactivity series → reaktivni niz
Reactivity series or activity series is a series of elements (usually metals) ranked by their reactivity degree, made for comparison of reactions of elements with other substances, e.g. acids and oxygen.
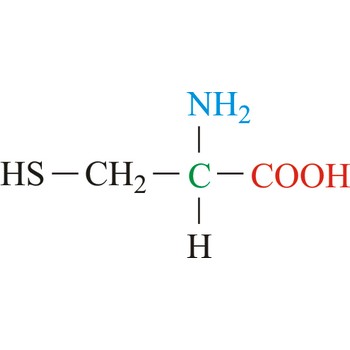
cysteine → cistein
Cysteine is neutral amino acids with polar side chains. Because of its high reactivity, the thiol group of cysteine has numerous biological functions. It serves as a potent nucleophile and metal ligand (particularly for iron and zinc), but is best known for its ability to form disulfide bonds, which often make an important contribution to the stability of extracellular proteins. Cysteine is a non-essential amino acid, which means that it is biosynthesized in humans.
- Abbreviations: Cys, C
- IUPAC name: 2-amino-3-sulfanylpropanoic acid
- Molecular formula: C3H7NO2S
- Molecular weight: 121.16 g/mol
halogens → halogeni elementi
Halogens are the elements fluorine (F) chlorine (Cl), bromine (Br), iodine (I), and astatine (At). They are non-metals, and make up part of the 17 group in the periodic table. Compounds of these elements are called halogenides or halides.
The halogens all have a strong unpleasant odour and will burn flesh. They do not dissolve well in water. The five elements are strongly electronegative. They are oxidising agents, with fluorine being the strongest and astatine being the weakest. They react with most metals and many non-metals.
Halogens form molecules which consist of atoms covalently bonded. With increasing atomic weight there is a gradation in physical properties. For example: Fluorine is a pale green gas of low density. Chlorine is a greenish-yellow gas 1.892 times as dense as fluorine. Bromine is a deep reddish-brown liquid which is three times as dense as water. Iodine is a grayish-black crystalline solid with a metallic appearance. And astatine is a solid with properties which indicate that it is somewhat metallic in character.
Citing this page:
Generalic, Eni. "Reaktivnost." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table