acid-base indicator → kiselo-bazni indikator
Acid-base indicator is a weak acid or weak base, such as litmus, methyl orange or phenolphthalein, which changes colour when it gains or loses an H+ ion.
acylaction reaction → reakcije aciliranja
Acylaction reaction involves the introduction of an acyl group (RCO-) into a compound. An alkyl halide is reacted with an alcohol or a carboxylic acid anhydride e.g.
The introduction of an acetyl group (CH3CO-) is acetylation, a process used for protecting -OH groups in organic synthesis.
amino acids → aminokiseline
Amino acids are compounds containing both a carboxylic acid group (-COOH) and an amino group (-NH2 ). The most important are the α-amino acids, in which the -NH2 group in attached to the C atom adjacent to the -COOH group. In the β-amino acids, there is an intervening carbon atom.
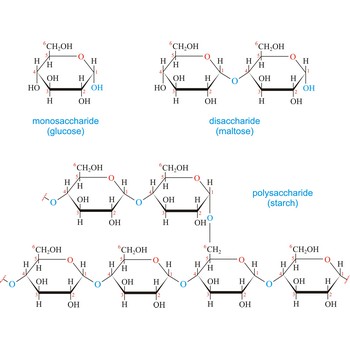
carbohydrate → ugljikohidrat
Carbohydrates (often called carbs for short) are polyhydroxy aldehydes or ketones, or substances that yield such compounds on hydrolysis. They are also known as saccharides, a term derived from the Latin word saccharum for sugar. Carbohydrates are the most abundant class of compounds in the biological world, making up more than 50 % of the dry weight of the Earth’s biomass. Every type of food we eat can have its energy traced back to a plant. Plants use carbon dioxide and water to make glucose, a simple sugar, in photosynthesis. Other carbohydrates such as cellulose and starch are made from the glucose. Light from the sun is absorbed by chlorophyll and this is converted to the energy necessary to biosynthesize carbohydrates
The term carbohydrate was applied originally to monosaccharides, in recognition of the fact that their empirical composition can be expressed as Cx(H2O)y. Later structural studies revealed that these compounds were not hydrates but the term carbohydrate persists.
Carbohydrates are generally classed as either simple or complex. Simple sugars, or monosaccharides, are carbohydrates that can’t be converted into smaller subunits by hydrolysis. Complex carbohydrates are made of two (disaccharides) or more (oligosaccharides, polysaccharides) simple sugars linked together by acetal (glycosidic) bonds and can be split into the former by hydrolysis.
ether → eter
Ethers are organic compounds with a formula R-O-R, where R is not equal to H. They may be derived from alcohols by elimination of water, but the major method is catalytic hydration of olefins. They are volatile highly flammable compounds; when containing peroxides they can detonate on heating. The term ether is often used synonymously with diethyl ether.
hydrogen → vodik
Hydrogen was discovered by Sir Henry Cavendish (England) in 1766. The origin of the name comes from the Greek words hydro and genes meaning water and generate. It is colourless, odourless gas, burns and forms explosive mixtures in air. Reacts violently with oxidants. Hydrogen is the most abundant element in the universe. Commercial quantities of hydrogen are produced by reacting superheated steam with methane or carbon. In lab work from reaction of metals with acid solutions or electrolysis. Most hydrogen is used in the production of ammonia and in metal refining. Also used as fuel in rockets. Its two heavier isotopes (deuterium and tritium) used respectively for nuclear fusion.
Kjeldahl’s method → Kjeldahlov postupak
Kjeldahl’s method is an analytical method for determination of nitrogen in certain organic compounds. The method was developed by the Danish chemist Johan Kjeldahl (1849-1900).
It involves addition of a small amount of anhydrous potassium sulphate to the test compound, followed by heating the mixture with concentrated sulphuric acid, often with a catalyst such as copper sulphate. As a result ammonia is formed. After alkalyzing the mixture with sodium hydroxyde, the ammonia is separated by distillation, collected in standard acid, and the nitrogen determined by back-titration.
- Kjeldahl flask for decomposition (500 ml – macro or 100 ml - micro)
- funnel for alkaline solution
- Wagner tube (drop catcher)
- condenser
- absorption flask with known volume of standard acid
mercury → živa
Mercury has been known since ancient times. The origin of the name comes from the Latin word hydrargyrum meaning liquid silver. It is heavy, silver-white metal, liquid at ordinary temperatures. Stable in air and water. Unreactive with alkalis and most acids. Gives off poisonous vapour. Chronic cumulative effects. Mercury only rarely occurs free in nature. The chief ore is cinnabar or mercury sulfide (HgS). Used in thermometers, barometers and batteries. Also used in electrical switches and mercury-vapour lighting products.
nitrogen → dušik
Nitrogen was discovered by Daniel Rutherford (Scotland) in 1772. The origin of the name comes from the Greek words nitron genes meaning nitre and forming and the Latin word nitrum (nitre is a common name for potassium nitrate, KNO3). It is colourless, odourless, generally inert gas. Minimally reactive at room temperature. A component of many organic and inorganic compounds. Makes up about 78 % of earth’s atmosphere. Nitrogen is obtained from liquid air by fractional distillation. Primarily to produce ammonia and other fertilizers. Also used in making nitric acid, which is used in explosives. Also used in welding and enhanced oil recovery.
Citing this page:
Generalic, Eni. "Organska kiselina." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table