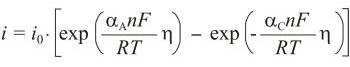analytical balance → analitička vaga
Analytical balances are instruments used for precise determining mass of matter. Analytical balances are sensitive and expensive instruments, and upon their accuracy and precision the accuracy of analysis result depends. The most widely used type of analytical balances are balances with a capacity of 100 g and a sensitivity of 0.1 mg. Not one quantitative chemical analysis is possible without usage of balances, because, regardless of which analytical method is being used, there is always a need for weighing a sample for analysis and the necessary quantity of reagents for solution preparation.
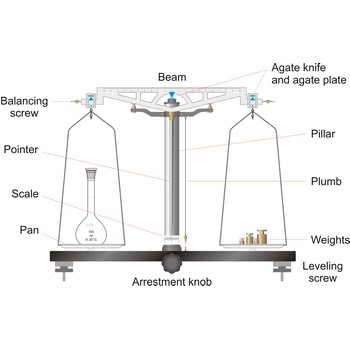
The working part of the balance is enclosed in a glass-fitted case. The baseplate is usually of black glass or black slate. The beam has agate knife-edges at its extremes, supporting stirrups from which balance pans are suspended. Another agate or steel knife-edge is fixed exactly in the middle of the beam on its bottom side. This knife-edge faces downwards and supports the beam. When not in use and during loading or unloading of the pans, the balance should be arrested.
The principle of operation of a modern laboratory balance bears some resemblance to its predecessor - the equal arm balance. The older instrument opposed the torque exerted by an unknown mass on one side of a pivot to that of an adjustable known weight on the other side. When the pointer returned to the center position, the torques must be equal, and the weight was determined by the position of the moving weights.
Modern electronic laboratory balances work on the principle of magnetic force restoration. In this system, the force exerted by the object being weighed is lifted by an electromagnet. A detector measures the current required to oppose the downward motion of the weight in the magnetic field.
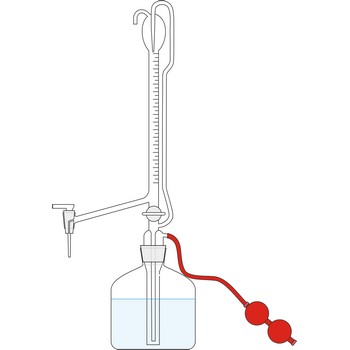
automatic burette → automatska bireta
Automatic burette is used for series of tests. It is connected with a bottle which contains the titration solution. The air is pumped into the bottle by a small rubber air pump, created the pressure in the bottle which the rises the solution to the top of burette. When the the burette is full, the valve is released, the pressure in the bottle falls and the burette automatically sets itself to zero. Work with automatic burettes is by far faster and the consumption of standard solution is smaller.
lattice energy → energija kristalne rešetke
Lattice energy is the energy per ion pair required to separate completely the ions in a crystal lattice at a temperature of absolute zero.
angular velocity → kutna brzina
A point-like object that undergoes circular motion changes its angular position from initial Θi to final Θf, relative to a fixed axis, specified in a coordinate system with an origin that coincides the centre of the circular path of object. The change in its angular position is called angular displacement ΔΘ = Θf - Θi. Also, a rigid body that rotates about a specified rotation axis, changing its angular position from initial Θi to final Θf, undergoes an angular displacement ΔΘ.
The average angular velocity, ωav, is the ratio of the angular displacement and the time interval Δt=tf-ti, in which that displacement occurs.
Θf and Θi are the initial and final angular position, respectively.
The instantaneous angular velocity ω is the limit of the average angular velocity, as Δt is made to approach zero.
ωav and ω are positive for the counterclockwise rotation (in direction of increasing Θ) and negative for the clockwise rotation (in direction of decreasing Θ).
SI unit for angular velocity is s-1.The measure for the angle Θ is radian. The relationship between radians and degrees is:
For example, the angular velocity of the minute hand of a clock is:
balance → vaga
Balance is an instrument to measure the mass (or weight) of a body. Balance beam type scales are the oldest type and measure weight using a fulcrum or pivot and a lever with the unknown weight placed on one end of the lever, and a counterweight applied to the other end. When the lever is balanced, the unknown weight and the counterweight are equal. The equal-arm balance consists of two identical pans hung from either end of a centrally suspended beam. The unequal-arm balance is made with one arm of the balance much longer than the other.
More modern substitution balances use the substitution principle. In this calibrated weights are removed from the single lever arm to bring the single pan suspended from it into equilibrium with a fixed counter weight. The substitution balance is more accurate than the two-pan device and enables weighing to be carried out more rapidly.
Electromagnetic force restoration balances also use a lever system but a magnetic field is used to generate the force on the opposite end of the lever and balance out the unknown mass. The current used to drive the magnetic coil is proportional to the mass of the object placed on the platform.
Butler-Volmer equation → Butler-Volmerova jednadžba
Butler-Volmer equation is an activation controlled reaction, the one for which the rate of reaction is controlled solely by the rate of the electrochemical charge transfer process, which is in turn an activation-controlled process. This gives rise to kinetics that are described by the Butler-Volmer equation:
where io is exchange current density, η is overpotential (η = E - Eo), n is number of electrons, αA is anodic transfer coefficient, and αC is cathodic transfer coefficient
Celsius temperature scale → Celsiusova temperaturna skala
For value of zero in Celsius temperature scale the freezing point of water at a pressure of 101 325 Pa is taken. The boiling point of water at a pressure of 101 325 Pa is taken as another reference point. This range is divided into 100 equal parts, and each part is an equivalent to 1 °C. Units of Celsius temperature scale (°C) and thermodynamic temperature scale (K) are identical
1 °C = 1 K.
chemical equation → kemijska jednadžba
Chemical equation is a way of denoting a chemical reaction using the symbol for the participating particles (atoms, molecules, ions, etc.); for example,
The single arrow is used for an irreversible reaction; double arrows are used for reversible reactions. When reactions involve different phases, it is usual to put the phase in brackets after the symbol.
| s | = | solid |
| l | = | liquid |
| g | = | gas |
| aq | = | aqueous |
The numbers a, b, c, and d, showing the relative numbers of molecules reacting, are called the stoichiometric coefficients. The convention is that stoichiometric coefficients are positive for reactants and negative for products. If the sum of the coefficients is zero, the equation is balanced.
electrochemical series → elektrokemijski niz
Electrochemical series is a series of chemical elements arranged in order of their standard electrode potentials. The hydrogen electrode
is taken as having zero electrode potential. An electrode potential is, by definition, a reduction potential.
Elements that have a greater tendency than hydrogen to lose electrons to their solution are taken as electropositive; those that gain electrons from their solution are below hydrogen in the series and are called electronegative.
The series shows the order in which metals replace one another from their salts; electropositive metals will replace hydrogen from acids.
Citing this page:
Generalic, Eni. "Nula." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table