hydrophobic interaction → hidrofobne interakcije
Hydrophobic interaction is the tendency of hydrocarbons (or of lipophilic hydrocarbon-like groups in solutes) to form intermolecular aggregates in an aqueous medium, and analogous intramolecular interactions. The name arises from the attribution of the phenomenon to the apparent repulsion between water and hydrocarbons. Use of the misleading alternative term hydrophobic bond is discouraged.
lateral chain → postranični lanac
Lateral chain is a shorter chain of hydrocarbons which is connected to the main chain of hydrocarbon.
monosaccharide → monosaharid
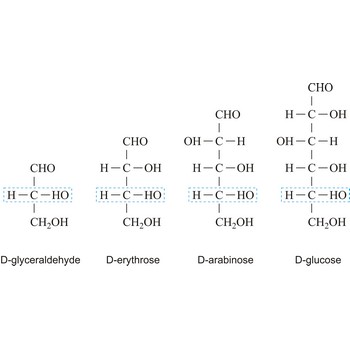
Monosaccharides are carbohydrates, with the general formula Cn(H2O)n, that cannot be decomposed to a simpler carbohydrates by hydrolysis.
Depending on whether the molecule contains an aldehyde group (-CHO) or a ketone group (-CO-) monosaccharide can be a polyhydroxy aldehyde (aldose) or a polyhydroxy ketone (ketose). These aldehyde and ketone groups confer reduction properties on monosaccharides. They are also classified according to the number of carbon atoms they contain: trioses have three carbon atoms, tetroses four, pentoses five, hexoses six, heptoses seven, etc. These two systems of classification are often combined. For example, a six-carbon polyhydroxy aldehyde such as D-glucose is an aldohexose, whereas a six-carbon polyhydroxy ketone such as D-fructose is a ketohexose.
The notations D and L are used to describe the configurations of carbohydrates. In Fischer projections of monosaccharides, the carbonyl group is always placed on top (in the case of aldoses) or as close to the top as possible (in the case of ketoses). If the OH group attached to the bottom-most asymmetric carbon (the carbon that is second from the bottom) is on the right, then the compound is a D-sugar. If the OH group is on the left, then the compound is an L-sugar. Almost all sugars found in nature are D-sugars.
Monosaccharides can exist as either straight-chain or ring-shaped molecules. During the conversion from straight-chain form to cyclic form, the carbon atom containing the carbonyl oxygen, called the anomeric carbon, becomes a chiral center with two possible configurations (anomers), α and β. When the stereochemistry of the first carbon matches the stereochemistry of the last stereogenic center the sugar is the α-anomer when they are opposite the sugar is the β-anomer.
polystyrene → polistiren
Polystyrene is a vinyl polymer. Structurally, it is a long hydrocarbon chain, with a phenyl group attached to every other carbon atom. Polystyrene is produced by free radical vinyl polymerization, from the monomer styrene. Polystyrene or Styrofoam is used in the construction industry as insulating material and for production of containers.
saturated fatty acid → zasićena masna kiselina
Saturated fatty acid is a fatty acid carrying the maximum possible number of hydrogen atoms (It doesn’t have any double bounds in the alkyl chain). The most important of these are:
| Butyric (butanoic acid) | CH3(CH2)2COOH |
| Lauric (dodecanoic acid) | CH3(CH2)10COOH |
| Myristic (tetradecanoic acid) | CH3(CH2)12COOH |
| Palmitic (hexadecanoic acid) | CH3(CH2)14COOH |
| Stearic (octadecanoic acid) | CH3(CH2)16COOH |
| Arachidic (eicosanoic acid) | CH3(CH2)18COOH |
seawater → more
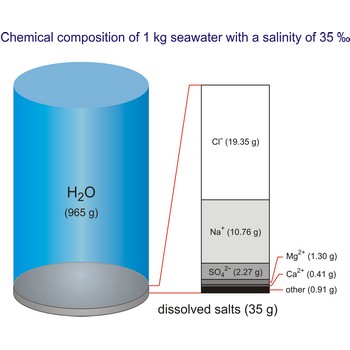
Seawater is a complex mixture of 96.5 % water, 3.5 % salts, and smaller amounts of other substances, including dissolved inorganic and organic materials, particulates, and a few atmospheric gases. The world's oceans cover nearly 71 % (361 840 000 km2) of the Earth's surface (510 100 000 km2), with an average depth of 3 682.2 m.
The density of seawater is higher than that of fresh water because of its higher salinity. Seawater's freezing point is lower than that of pure water and its boiling point is higher. The average salinity of the ocean is 35 ‰, which means that for every kilograms of water, there are 35 g of salt. The relative abundance of the major salts in seawater are constant regardless of the ocean. Only six elements and compounds comprise about 99 % of sea salts: chlorine (Cl-), sodium (Na+), sulfur (SO42-), magnesium (Mg2+), calcium (Ca2+), and potassium (K+).
smoke → dim
Smoke is a fine suspension of solid particles in a gas. In general smoke particles range downward from about 5 μm in diameter to less than 01 μm in diameter. Smoke generally refers to a visible mixture of products given off by the incomplete combustion of an organic substance such as wood, coal, fuel oil etc. This airborne mixture general contains small particles (dusts) of carbon, hydrocarbons, ash etc. as well as vapors such as carbon monoxide, carbon dioxide, and water vapor.
Citing this page:
Generalic, Eni. "Nezasićeni ugljikovodik." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table