Results 1–10 of 10 for negativan pol
negative pole → negativan pol
Negative pole is that half-cell in electrochemical cell that has the most negative electrode potential.
chemical equation → kemijska jednadžba
Chemical equation is a way of denoting a chemical reaction using the symbol for the participating particles (atoms, molecules, ions, etc.); for example,
The single arrow is used for an irreversible reaction; double arrows are used for reversible reactions. When reactions involve different phases, it is usual to put the phase in brackets after the symbol.
| s | = | solid |
| l | = | liquid |
| g | = | gas |
| aq | = | aqueous |
The numbers a, b, c, and d, showing the relative numbers of molecules reacting, are called the stoichiometric coefficients. The convention is that stoichiometric coefficients are positive for reactants and negative for products. If the sum of the coefficients is zero, the equation is balanced.
enthalpy → entalpija
Enthalpy (H) is a thermodynamic property of a system defined by
where U is the internal energy of the system, p its pressure, and V its volume. J.W. Gibbs put the concept of an ensemble forward in 1902. In a chemical reaction carried out in the atmosphere the pressure remains constant and the enthalpy of reaction (ΔH), is equal to
For an exothermic reaction ΔH is taken to be negative.
Gibbs free energy → Gibbsova slobodna energija
Gibbs free energy (G) is an important function in chemical thermodynamics, defined by
where H is the enthalpy, S the entropy, and T the thermodynamic temperature. Gibbs free energy is the energy liberated or absorbed in a reversible process at constant pressure and constant temperature. Sometimes called Gibbs energy and, in older literature, simply free energy.
Changes in Gibbs free energy, ΔG, are useful in indicating the conditions under which a chemical reaction will occur. If ΔG is negative the reaction will proceed spontaneously to equilibrium. In equilibrium position ΔG = 0.
global warming → globalno zatopljenje
Global warming or greenhouse effect is an effect occurring in the atmosphere because of the presence of certain gases (greenhouse gases) that absorb infrared radiation. Light and ultraviolet radiation from the sun is able to penetrate the atmosphere and warm the Earth’s surface. This energy is re-radiated as infrared radiation which because of its longer wavelength, is absorbed by such substances as carbon dioxide. The overall effect is that the average temperature of the Earth and its atmosphere is increasing (so-called global Warming). The effect is similar to that occurring in a greenhouse, where light and long-wavelength ultraviolet radiation can pass through the glass into greenhouse but the infrared radiation is absorbed by the glass and part of it is re-radiated into the greenhouse.
The greenhouse effect is seen as a major environmental hazard. Average increases in temperature could change weather patterns and agricultural output. It might also lead to melting of the polar ice caps and a corresponding rise in sea level. Carbon dioxide, from fossil-fuel power stations and car exhausts, is the main greenhouse gas. Other contributory pollutants are nitrogen oxides, ozone, methane, and chloroflourocarbons.
hydrogen bond → vodikova veza
Hydrogen is a bond formed by a hydrogen atom to an electronegative atom, and is denoted by dashed lines H-X---H-B. A hydrogen atom covalently bound to an oxygen (electronegative atom) has a significant positive charge and can form a weak bond to another electronegative atom.
oxidation number → oksidacijski broj
Atoms can give one or more electrons for bond forming. The valence of any atom, which comes from stechiometrical relation of interbonded atoms, is called an oxidation number or an oxidation degree. Oxidation number of atoms in elementary state is zero. An atom of greater electronegativity has a negative, and an atom of lesser electronegativity has a positive oxidation number.
stoichiometric coefficient → stehiometrijski koeficijent
Stoichiometric coefficient (ν) is the number appearing before the symbol for each compound in the equation for a chemical reaction. By convention, it is negative for reactants and positive for products.
Stoichiometric coefficients describe the stoichiometry of the chemical reaction.
In this equation, a, b, c and d are called as Stoichiometric coefficients of the A, B, C and D respectively.
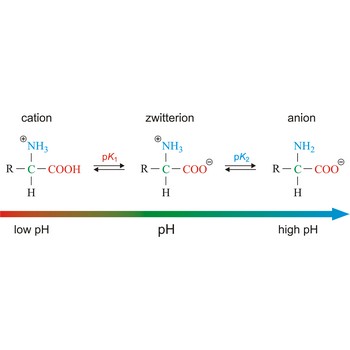
zwitterion → dipolarni ion
Zwitterion, also known as inner salt or dipolar ion, is an ion with a positive and a negative electrical charge at different locations within a molecule. As the molecule contains two opposite charges, it is electrically neutral. The term zwitterion is derived from the German word zwitter, meaning a hybrid, hermaphrodite. Zwitterions can be formed from compounds that contain both acid groups and base groups in their molecules (ampholytes).
All of the common amino acids found in proteins are ampholytes because they contain a carboxyl group (-COOH) that acts as an acid and an amino group (-NH2) that acts as a base. In the solid state, amino acids exist in the dipolar or zwitterion form. If acid is added to a solution containing the zwitterion, the carboxylate group captures a hydrogen (H+) ion, and the amino acid becomes positively charged. If base is added, ion removal of the H+ ion from the amino group of the zwitterion produces a negatively charged amino acid.
Citing this page:
Generalic, Eni. "Negativan pol." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table