aluminate → aluminat
Aluminate is a salt formed when aluminium hydroxide or y-alumina is dissolved in solutions of strong bases, such as sodium hydroxide. Aluminates exist in solutions containing the aluminate ion, commonly written [Al(OH)4]-. In fact the ion probably is a complex hydrated ion and can be regarded as formed from a hydrated Al3+ ion by removal of four hydrogen ions:
Other aluminates and polyaluminates, such as [Al(OH)6]3- and [(HO)3AlOAl(OH)3]2-, are also present.
amphoteric substances → amfoterna tvar
Amphoteric substances can behave as acids or as bases depending upon their surroundings. So aluminium hydroxide in reaction with acids will behave as a base
and in reaction with bases it will act as an acid
Bravais lattice → Bravaisova rešetka
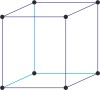
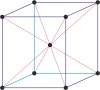
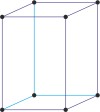
Bravais lattice is a set of points constructed by translating a single point in discrete steps by a set of basis vectors. The French crystallographer Auguste Bravais (1811-1863) established that in three-dimensional space only fourteen different lattices may be constructed. All crystalline materials recognised till now fit in one of these arrangements. The fourteen three-dimensional lattices, classified by crystal system, are shown to the bottom.
|
Crystal system
|
Bravais lattices
|
|||
|
cubic a=b=c α=β=γ=90° |
 |
 |
 |
|
|
|
simple cubic
|
body-centered cubic
|
face-centered cubic
|
|
|
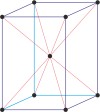
tetragonal a=b≠c α=β=γ=90° |
 |
 |
||
|
|
simple tetragonal
|
body-centered tetragonal
|
||
|
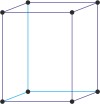
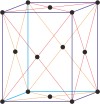
orthorhombic a≠b≠c α=β=γ=90° |
 |
 |
 |
 |
|
|
simple orthorhombic
|
base-centered orthorhombic
|
body-centered orthorhombic
|
face-centered orthorhombic
|
|
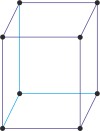
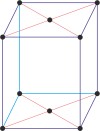
monoclinic a≠b≠c α=γ=90°≠β |
 |
 |
||
|
|
simple monoclinic
|
base-centered monoclinic
|
||
|
hexagonal a=b≠c α=β=90° γ=120° |
 |
|||
|
|
hexagonal
|
|||
|
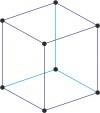
rhombohedral a=b=c α=β=γ≠90° |
 |
|||
|
|
rhombohedral
|
|||
|
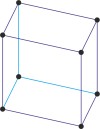
triclinic a≠b≠c α≠β≠γ≠90° |
 |
|||
|
triclinic
|
||||
buffer → pufer otopina
Buffer is a solution designed to maintain a constant pH when small amounts of a strong acid or base are added. Buffers usually consist of a fairly weak acid and its salt with a strong base. Suitable concentrations are chosen so that the pH of the solution remains close to the pKa of the weak acid.
carboxylates → karboksilati
Carboxylates is a common name for all salts that carboxylic acids yield by reacting with hydroxides, carbonates, bicarbonates and other alkaline reagents.
chlorination → kloriranje
1. Chlorination is an addition or substitution of chlorine in organic compounds.
2. Chlorination is a sterilisation of drinking and swimming pool water or oxidation of undesirable impurities, using chlorine or its compounds.
anhydrous → anhidrid
Anhydrous (without water) is an applied to minerals which do not contain water of crystallization or water of chemical combination. For example, strongly heated copper (II) sulphate pent hydrate (CuSO4•5H2O) produces anhydrous copper (II) sulphate (CuSO4). Less stable and more dangerous to use than hydrated.
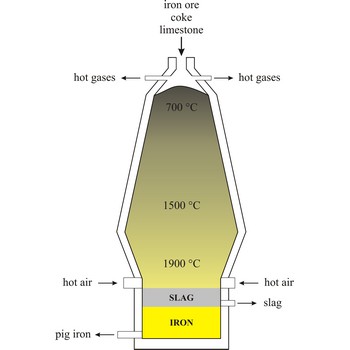
blast furnace → visoka peć
Blast furnace is a furnace for smelting of iron from iron oxide ores (hematite, Fe2O3 or magnetite, Fe3O4). Coke, limestone and iron ore are poured in the top, which would normally burn only on the surface. The hot air blast to the furnace burns the coke and maintains the very high temperatures that are needed to reduce the ore to iron. The reaction between air and the fuel generates carbon monoxide. This gas reduces the iron(III) oxide in the ore to iron.
Because the furnace temperature is in the region of 1500 °C, the metal is produced in a molten state and this runs down to the base of the furnace.
The production of iron in a blast furnace is a continuous process. The furnace is heated constantly and is re-charged with raw materials from the top while it is being tapped from the bottom. Iron making in the furnace usually continues for about ten years before the furnace linings have to be renewed.
deoxyribonucleic acid → dezoksiribonukleinska kiselina
Deoxyribonucleic acid (DNA) is a nucleic acid with 2-deoxy-D-ribose as the sugar in its nucleotides. DNA contains encoded genetic information, specifically templates for the synthesis of all of an organism’s proteins and enzymes.
DNA was first identified in the 1869 by Swiss chemist Friedrich Miescher (1844-1895). In 1953, American biologist James Dewey Watson (1928-) and English physicist Francis Harry Compton Crick (1916–2004) had discovered that DNA occurs in the cell as a double helix, with two long strands of the molecule wound around each other, and further that the chemical structure of the molecule dictates that adenine (A) always aligns or pairs with thymine (T), and cytosine (C) always pairs with guanine (G). It is this base pairing that allows DNA in a cell to copy itself, and transfer its information to a new cell. The diameter of the helix is 2.0 nm and there is a residue on each chain every 0.34 nm in the z direction. The angle between each residue on the same strand is 36°, so that the structure repeats after 10 residues (3.4 nm) on each strand.
Citing this page:
Generalic, Eni. "Konjugirana baza." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table