glycoside → glikozid
Glycoside is one of a group of organic compounds in which a sugar group is bonded through its anomeric carbon to another group via a glycosidic bond. The sugar group is known as the glycon and the non-sugar group as the aglycon. According to the IUPAC definition, all disaccharides and polysaccharides are glycosides where the aglycone is another sugar.
In the free hemiacetal form, sugars will spontaneously equilibrate between the α and β anomers. However, once the glycosidic bond is formed, the anomeric configuration of the ring is locked as either α or β. Therefore, the alpha and beta glycosides are chemically distinct. They will have different chemical, physical, and biological properties. Many glycosides occur abundantly in plants, especially as flower and fruit pigments.
The term glycoside was later extended to cover not only compounds in which the anomeric hydroxy group is replaced by a group -OR, but also those in which the replacing group is -SR (thioglycosides), -SeR (selenoglycosides), -NR1R2 (N-glycosides), or even -CR1R2R3 (C-glycosides). Thioglycoside and selenoglycoside are legitimate generic terms; however the use of N-glycoside, although widespread in biochemical literature, is improper and not recommended here (glycosylamine is a perfectly acceptable term). C-Glycoside is even less acceptable. All other glycosides are hydrolysable; the C-C bond of C-glycosides is usually not. The use and propagation of names based on C-glycoside terminology is therefore strongly discouraged.
half-life → vrijeme poluraspada
For a simple radioactive decay process, half-life, t1/2, is defined as the time required for the activity of a given radioactive isotopes to decrease to half its value by that process.
The half-life is a characteristic property of each radioactive isotope and is independent of its amount or condition.
hybridization → hibridizacija
Hybridization is an internal linear combination of atomic orbitals, in which the wave functions of the atomic orbitals are added together to generate new hybrid wave functions. The new orbitals which are formed are hybrids of the originals and have properties (shape, size and energy) that are somewhere in between.
ion exchanger → ionski izmjenjivač
Ion-exchanger is a solid or liquid material containing ions that are exchangeable with other ions with a like charge that are present in a solution in which the material is insoluble. Ion-exchange resins consist of various copolymers having a cross-linked three-dimensional structure to which ionic groups have been attached.
kinetic energy → kinetička energija
Kinetic energy (Ek) is associated with the state of motion of a body. It is a scalar property and defined to be
Kinetic energy is most clearly exhibited in gases, in which molecules have much greater freedom of motion than in liquids and solids.
metallic glass → metalno staklo
Certain alloys can solidify by extremely rapid cooling out of melt without formation of a crystal lattice, that is in the amorphous form - such, amorphous alloys are so called metallic glasses. The alloy of zirconium, beryllium, titanium, copper, and nickel is one of the first metallic glasses that can be made in bulk and formed into strong, hard, useful objects.
Unlike pure metals and most metal alloys, metallic glasses have no regular crystalline structure. This lack of long range order or microstructure is related to such desirable features as strength and low damping which is one reason why the premier use for zirconium-based metallic glass is in the manufacture of expensive golf club heads. Metallic glasses can be quite strong yet highly elastic, and they can also be quite tough (resistant to fracture). Even more interesting are the thermal properties; for instance, just like an oxide glass, there is a temperature (called the glass transition temperature) above which a metallic glass becomes quite soft and flows easily. This means that there are lots of opportunities for easily forming metallic glasses into complex shapes.
non-metal → nemetal
Non-metals are defined as elements that are not metals.
Their physical properties generally include:
- They are poor conductors.
- They are brittle, not ductile in their solid state.
- They show no metallic lustre.
- They may be transparent or translucent.
- They have low density.
- They form molecules which consists of atoms covalently bonded; the noble gases are monoatomic.
Their chemical properties are generally:
- They usually have four to eight valence electrons.
- They have high electron affinities (except the noble gases)
- They are good oxidising agents (except the noble gases)
- They have hydroxides which are acidic (except the noble gases)
- They are electronegative.
monosaccharide → monosaharid
Monosaccharides are carbohydrates, with the general formula Cn(H2O)n, that cannot be decomposed to a simpler carbohydrates by hydrolysis.
Depending on whether the molecule contains an aldehyde group (-CHO) or a ketone group (-CO-) monosaccharide can be a polyhydroxy aldehyde (aldose) or a polyhydroxy ketone (ketose). These aldehyde and ketone groups confer reduction properties on monosaccharides. They are also classified according to the number of carbon atoms they contain: trioses have three carbon atoms, tetroses four, pentoses five, hexoses six, heptoses seven, etc. These two systems of classification are often combined. For example, a six-carbon polyhydroxy aldehyde such as D-glucose is an aldohexose, whereas a six-carbon polyhydroxy ketone such as D-fructose is a ketohexose.
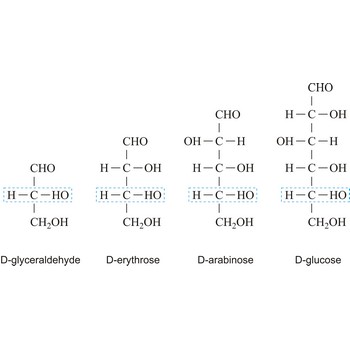
The notations D and L are used to describe the configurations of carbohydrates. In Fischer projections of monosaccharides, the carbonyl group is always placed on top (in the case of aldoses) or as close to the top as possible (in the case of ketoses). If the OH group attached to the bottom-most asymmetric carbon (the carbon that is second from the bottom) is on the right, then the compound is a D-sugar. If the OH group is on the left, then the compound is an L-sugar. Almost all sugars found in nature are D-sugars.
Monosaccharides can exist as either straight-chain or ring-shaped molecules. During the conversion from straight-chain form to cyclic form, the carbon atom containing the carbonyl oxygen, called the anomeric carbon, becomes a chiral center with two possible configurations (anomers), α and β. When the stereochemistry of the first carbon matches the stereochemistry of the last stereogenic center the sugar is the α-anomer when they are opposite the sugar is the β-anomer.
octet rule → pravilo okteta
Octet rule states that the chemical properties of the elements repeat on a regular basis with increasing atomic mass, and that the chemical properties of each eight element are similar. Since the inert gases, with the exception of helium have eight electrons in their outer shells, this stable electronic configuration is called the octet rule. In chemical reactions atoms of elements tend to react in such a way as to achieve the electronic configuration of the inert gas nearest to them in the periodic table. There are a number of exceptions to the octet rule.
Citing this page:
Generalic, Eni. "Koligativno svojstvo." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table