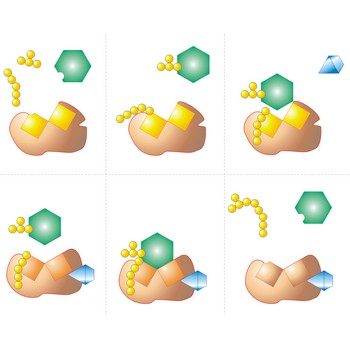
active site → aktivno mjesto
Active site is a pocket or crevice on an enzyme molecule that fits reactant molecules like a hand in a glove. The active site lowers the activation energy for reaction
angular velocity → kutna brzina
A point-like object that undergoes circular motion changes its angular position from initial Θi to final Θf, relative to a fixed axis, specified in a coordinate system with an origin that coincides the centre of the circular path of object. The change in its angular position is called angular displacement ΔΘ = Θf - Θi. Also, a rigid body that rotates about a specified rotation axis, changing its angular position from initial Θi to final Θf, undergoes an angular displacement ΔΘ.
The average angular velocity, ωav, is the ratio of the angular displacement and the time interval Δt=tf-ti, in which that displacement occurs.
Θf and Θi are the initial and final angular position, respectively.
The instantaneous angular velocity ω is the limit of the average angular velocity, as Δt is made to approach zero.
ωav and ω are positive for the counterclockwise rotation (in direction of increasing Θ) and negative for the clockwise rotation (in direction of decreasing Θ).
SI unit for angular velocity is s-1.The measure for the angle Θ is radian. The relationship between radians and degrees is:
For example, the angular velocity of the minute hand of a clock is:
sand filtration → pješčana filtracija
Sand filtration is a frequently used and very robust method of removing suspended solids from water. The filtration medium consists of a multiple layer of sand with a variety in size and specific gravity. Sand filters can be supplied in different sizes and materials, both hand operated and fully automatically.
benzene → benzen
Benzene is a colourless liquid hydrocarbon, C6H6, b.p. 80 °C. It is now made from petroleum by catalytic reforming (formerly obtained from coal tar). Benzene is the archetypal aromatic compound. It has an unsaturated molecule, yet will not readily undergo addition reactions. On the other hand, it does undergo substitution reactions in which hydrogen atoms are replaced by other atoms or groups.
In 1865, Friedrich August Kekulé purposed the benzene molecule structure as a hexagonal ring which consists of six carbon atoms with alternate carbon-carbon single and carbon-carbon double bond. But such a structure should be highly reactive, and so didn't account for the unreactive nature of benzene. We now know that the best representation for the structure of benzene is indeed, hexagonal, with each C-C bond distance being identical and intermediate between those for a single and double bond. The π-orbitals from each neighbouring carbon atom overlap to form a delocalised molecular orbital which extends around the ring, giving added stability and with it, decreased reactivity. That is the reason the structural formula of benzene represents as a hexagon with a circle in the center which represents the delocalized electrons.
beryllium → berilij
Beryllium was discovered by Friedrich Wöhler (Germany) and independently by A. B. Bussy (France) in 1828. The origin of the name comes from the Greek word beryllos meaning mineral beryl; also called glucinium from the Greek word glykys meaning sweet. It is steel-grey metal. It resists attack by concentrated nitric acid, has excellent thermal conductivity and is nonmagnetic. At ordinary temperatures, it resists oxidation in air. Beryllium and its salts are toxic and should be handled with the greatest of care. Beryllium is found mostly in minerals like beryl [AlBe3(Si6O18)] and chrysoberyl (Al2BeO4). Pure beryllium is obtained by chemically reducing beryl mineral. Also by electrolysis of beryllium chloride. Its ability to absorb large amounts of heat makes it useful in spacecraft, missiles, aircraft, etc. Emeralds are beryl crystals with chromium traces giving them their green colour.
chain reaction → lančana reakcija
Chain reaction is a reaction done in three steps: initiation in which usually radicals are made which react with other molecules in stage of propagation and, when all reactants are spent, it ends by termination when all available radicals are completely spent.
Nuclear chain reaction refers to a process in which neutrons released in fission produce an additional fission in at least one further nucleus. Nuclear power plants operate by precisely controlling the rate at which nuclear reactions occur. On the other hand, nuclear weapons are specifically engineered to produce a reaction that is so fast and intense it cannot be controlled after it has started.
Fischer-Tropsch process → Fischer-Tropschov postupak
Fischer-Tropsch process is an industrial method of making hydrocarbon fuels from carbon monoxide and hydrogen. The process was introduced in 1933. and used by Germany in World War II. to produce motor fuel. Hydrogen and carbon monoxide are mixed in the ratio 2:1 (water gas was used with added hydrogen) and passed at 200 °C over a nickel or cobalt catalyst. The resulting hydrocarbon mixture can be separated into a higher-boiling fraction for Diesel engines and a lower-boiling petrol fraction. The petrol fraction contains a high proportion of straight-chain hydrocarbons and has to be reformed for use in motor fuel. Alcohols, aldehydes, and ketones are also present. The process is also used in the manufacture of SNG from coal. It is named after the German chemist Franz Fischer (1852-1932) and the Czech Hans Tropsch (1839-1935).
Geiger counter → Geigerov brojač
Geiger counter (Geiger-Muller counter) is a device used to detect and measure ionising radiation. It consists of a tube containing a low-pressure gas (usually argon or neon with methane) and a cylindrical hollow cathode through the centre of which runs a fine-wire anode. A potential difference of about 1 000 V is maintained between the electrodes. An ionising particle or photon passing through a window into the tube will cause an ion to be produced and the high potential will accelerate it towards its appropriate electrode, causing an avalanche of further ionisations by collision. The consequent current pulses can be counted in electronic circuits or simply amplified to work a small loudspeaker in the instrument. It was first devised in 1908 by the German physicist Hans Geiger (1882-1945). Geiger and W. Muller produced an improved design in 1928.
Goldschmidt process → Goldschmidtov postupak
Goldschmidt process (thermite process) is a method of extracting metals by reducing the oxide with aluminium powder. Practically all the metallic oxides are reducible by this method, the chief exception being the oxide of magnesium. The thermite process was developed by the German chemist Hans Goldschmidt (1861-1923) in 1893.
Goldschmidt was originally interested in producing very pure metals, but he soon realized the value in welding, a process known as Thermit welding.
Citing this page:
Generalic, Eni. "Hans hamberger hill." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table