platinum → platina
Platinum was discovered by Antonio de Ulloa (South America) in 1735. The origin of the name comes from the Spanish word platina meaning silver. It is rare, very heavy, soft, silvery-white metal. Resists oxygen and water. Platinum is produced from deposits of native, or elemental. Used in jewellery, to make crucible and special containers and as a catalyst. Used with cobalt to produce very strong magnets. Also to make standard weights and measures. Resists corrosion and acid attacks except aqua regia.
polymorphism → polimorfija
Polymorphism is the ability of a solid substance to crystallise into more than one different crystal structure. Different polymorphs have different arrangements of atoms within the unit cell, and this can have a profound effect on the properties of the final crystallised compound. The change that takes place between crystal structures of the same chemical compound is called polymorphic transformation.
The set of unique crystal structures a given compound may form are called polymorphs. Calcium carbonate is dimorphous (two forms), crystallizing as calcite or aragonite. Titanium dioxide is trimorphous; its three forms are brookite, anatase, and rutile. The prevailing crystal structure depends on both the temperature and the external pressure.
Iron is a metal with polymorphism structure. Each structure stable in the range of temperature, for example, when iron crystallizes at 1 538 °C it is bcc (δ-iron), at 1 394 °C the structure changes to fcc (γ-iron or austenite), and at 912 °C it again becomes bcc (α-iron or ferrite).
Polymorphism of an element is called allotropy.
proton → proton
Proton is a stable elementary particle of unit positive charge and spin 1/2. Protons and neutrons, which are collectively called nucleons, are the constituents of the nucleus.
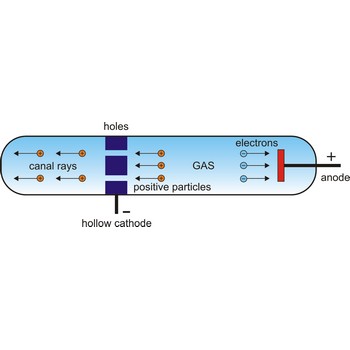
In 1886, German physicist Eugene Goldstein (1850-1930) discovered positive particles by using a modified Crookes tube with holes in the cathode in an evacuated tube. When cathode rays were given off in one direction toward the anode, other rays found their way through the holes in the cathode and sped off in the opposite direction. Since these other rays traveled in the direction opposite to the negatively charged cathode rays, it seemed that they must be composed of positively charged particles. Rutherford suggested that this fundamental positive particle be called the proton.
radioactive indicator → radioaktivni indikator
By use of suitable radioactive isotopes biochemical processes can be observed in plants, animals and humans, by measuring radioactive radiation of radioactive indicator. Artificial radioactive isotopes have the same chemical properties as natural ones, which enable us to mark those natural isotopes with addition of artificial ones and in this way follow the path of those elements during a chemical reaction. One of the most important radioactive indicators is the radioactive carbon 14C.
radon → radon
Radon was discovered by Friedrich Ernst Dorn (Germany) in 1900. The origin of the name is variation of the name of element radium; radon was called niton at first, from the Latin word nitens meaning shining. It is colourless, odourless radioactive, heavy, noble gas. Chemically inert and non-flammable. Highly radiotoxic. Carcinogen by inhalation. Radon is formed from the decay of radium in the earths crust. Used to treat some forms of cancer.
rotational inertia → moment tromosti
Rotational inertia of a body is defined as
for a system of discrete particles (each of mass mi), and as
for a body with continuously distributed mass (dm is the mass element). ri and r represent the perpendicular distance from the axis of rotation to the mass element of the body.
SI unit for rotational inertia is kg m2.
scandium → skandij
Scandium was discovered by Lars Fredrik Nilson (Sweden) in 1879. The origin of the name comes from the Latin word Scandia meaning Scandinavia. It is fairly soft, silvery-white metal. Burns easily. Tarnishes readily in air. Reaction with water releases hydrogen. Reacts with air and halogens. Scandium occurs mainly in the minerals thortveitile (~34 % scandium) and wiikite. Also in some tin and tungsten ores. Pure scandium is obtained as a by-product of uranium refining. Scandium metal is used in some aerospace applications. Scandium oxide (Sc2O2) is used in the manufacture of high-intensity electric lamps. Scandium iodide (ScI3) is used in lamps that produce light having a colour closely matching natural sunlight.
seawater → more
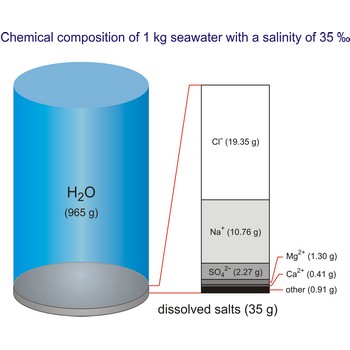
Seawater is a complex mixture of 96.5 % water, 3.5 % salts, and smaller amounts of other substances, including dissolved inorganic and organic materials, particulates, and a few atmospheric gases. The world's oceans cover nearly 71 % (361 840 000 km2) of the Earth's surface (510 100 000 km2), with an average depth of 3 682.2 m.
The density of seawater is higher than that of fresh water because of its higher salinity. Seawater's freezing point is lower than that of pure water and its boiling point is higher. The average salinity of the ocean is 35 ‰, which means that for every kilograms of water, there are 35 g of salt. The relative abundance of the major salts in seawater are constant regardless of the ocean. Only six elements and compounds comprise about 99 % of sea salts: chlorine (Cl-), sodium (Na+), sulfur (SO42-), magnesium (Mg2+), calcium (Ca2+), and potassium (K+).
specific weight → specifična težina
Specific weight (γ) is defined as the ratio between the weight of a mass element, Δm, and the volume, ΔV, occupied by that element. As density (average) is defined as the ratio of a mass element and its volume, specific weight is equal to:
where g is gravitational acceleration.
spin → spin
Spin is the intrinsic angular momentum of an elementary particle, or system of particles such as nucleus, that is also responsible for the magnetic moment; or, a particle or nucleus possessing such a spin. The spins of nuclei have characteristic fixed values. Pairs of neutrons and protons align to cancel out their spins, so that nuclei with an odd number of neutrons and/or protons will have a net non-zero rotational component characterized by a non-zero quantum nuclear spin number.
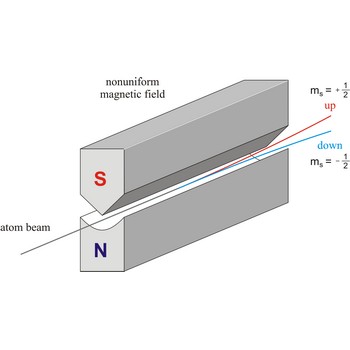
Stern-Gerlach experiment: a beam of silver atoms is split into two beams when it traverses a nonuniform magnetic field. Atoms with spin quantum number ms=+1/2 follow one trajectory, and those with ms=+1/2 follow another.
Citing this page:
Generalic, Eni. "Halogeni elementi." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table