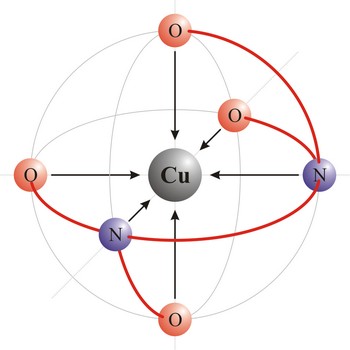
octahedral molecular geometry → oktaedarska geometrija molekule
Octahedral molecular geometry (square bipyramidal shape) describes the shape of compounds where six atoms or ligands are symmetrically arranged around a central atom. The sulfur hexafluoride (SF6), with six bonding pairs, is predicted and found to be a regular octahedron. Four of the attachments are positioned in a square plane with 90° bond angles. The remaining two attachments are positioned perpendicular (90°) to the square plane at opposite ends of the central atom. Molecules with an octahedral electron pair geometries have sp3d2 (or d2sp3) hybridization at the central atom.
polydentant ligand → polidentantni liganad
Polydentant ligands contain more co-ordination points (can give more electron pairs) and they form complex ringlike structures (celate complexes) by replacing two or more monodentant ligands. That kind of ligand is EDTA which has 6 co-ordinational points and with metals it creates complexes, always in 1:1 ratio.
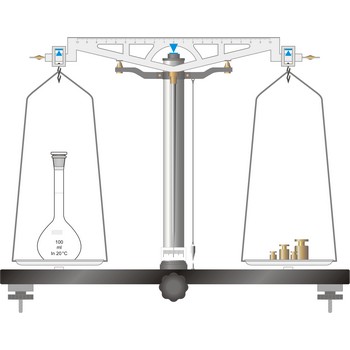
precision balance → tehnička vaga
Precision balances typically display results from three to one decimal places (0.001 g up to 0.1 g). The readability precision balances are reduced when compared to analytical balances but, precision balances accommodate higher capacities (up to several kilograms). In its traditional form, it consists of a pivoted horizontal lever of equal length arms, called the beam, with a weighing pan, also called scale, suspended from each arm.
In electronic top pan, or toploader balances, mass is determined not by mechanical deflection but by electronically controlled compensation of an electric force. The signal generated enables the mass to be read from a digital display. The mass of the empty container can be stored in the balance’s computer memory and automatically deducted from the mass of the container plus its contents.
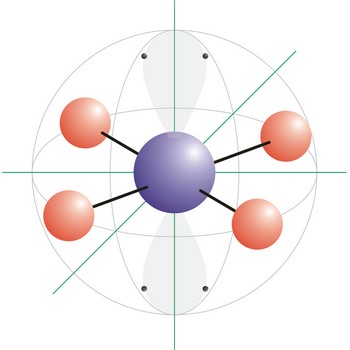
square planar molecular geometry → kvadratna planarna geometrija molekule
Square planar is a molecular shape that results when there are four bonds and two lone pairs on the central atom in the molecule. An example of a square planar molecule is xenon tetrafluoride (XeF4). This molecule is made up of six equally spaced sp3d2 (or d2sp3) hybrid orbitals arranged at 90° angles. The shape of the orbitals is octahedral. Two orbitals contain lone pairs of electrons on opposite sides of the central atom. The remaining four atoms connected to the central atom give the molecule a square planar shape.
square pyramidal molecular geometry → kvadratna piramidalna geometrija molekule
Square pyramidal is a molecular shape that results when there are five bonds and one lone pair on the central atom in the molecule. Bromine pentafluoride (BrF5) has the geometry of a square pyramid, with fluorine atoms occupying five vertices, one of which is above the plane of the other four. This molecule is made up of six equally spaced sp3d2 (or d2sp3) hybrid orbitals arranged at 90° angles. The shape of the orbitals is octahedral. Because of the high symmetry of the octahedral arrangement, all six positions are equivalent, so it does not matter in which position in the drawing we put the lone pair. The remaining four atoms connected to the central atom give the molecule a square planar shape.
solar cell → sunčeva ćelija
Solar cell, or photovoltaic cell, is a device that captures sunlight and transforms it directly to electricity. All solar cells make use of photovoltaic effect, so often they are called photovoltaic cells. Almost all solar cells are built from solid-state semiconducting materials, and in the vast majority of these the semiconductor is silicon.
The photovoltaic effect involves the generation of mobile charge carriers-electrons and positively charged holes-by the absorption of a photon of light. This pair of charge carriers is produced when an electron in the highest filled electronic band of a semiconductor (the valence band) absorbs a photon of sufficient energy to promote it into the empty energy band (the conduction band). The excitation process can be induced only by a photon with an energy corresponding to the width of the energy gap that separates the valence and the conduction band. The creation of an electron-hole pair can be converted into the generation of an electrical current in a semiconductor junction device, wherein a layer of semiconducting material lies back to back with a layer of either a different semiconductor or a metal. In most photovoltaic cells, the junction is p-n junction, in which p-doped and n-doped semiconductors are married together. At the interface of the two, the predominance of positively charged carriers (holes) in the p-doped material and of negatively charged carriers (electrons) in the n-doped material sets up an electric field, which falls off to either side of the junction across a space-charge region. When absorption of a photon in this region generates an electron-hole pair, these charge carriers are driven in opposite directions by the electric field, i.e. away from the interface and toward the top and bottom of the two-layer structure, where metal electrodes on these faces collect the current. The electrode on the top layer (through which light is absorbed) is divided into strips so as not to obscure the semiconducting layers below. In most widely used commercial solar cells, the p-doped and n-doped semiconductive layers are formed within a monolithic piece of crystalline silicon. Silicon is able to absorb sunlight at those wavelengths at which it is most intense-from the near-infrared region (wavelengths of around 1200 nm) to the violet (around 350 nm).
superoxide → superoksid
Superoxides are binary compounds containing oxygen in the -½ oxidation state. Sodium superoxide (NaO2) can be prepared with high oxygen pressures, whereas the superoxides of rubidium, potassium, and cesium can be prepared directly by combustion in air. These compounds are yellow to orange paramagnetic solids. Superoxide ion, O2-, has an unpaired electron, is not particularly stable, and spontaneously decomposes into peroxide over time.
They are strong oxidising agents that vigorously hydrolyze (react with water) to produce superoxide and oxygen gas.
T-shaped molecular geometry → T-oblik geometrije molekule
T-shape is a molecular geometry that results when there are 3 bonds and 2 lone pairs around the central atom in the molecule. The atoms bonded to the central atom lie at the ends of a T with 90° angles between them. Molecules with an trigonal bipyramidal electron pair geometries have sp3d (or dsp3) hybridization at the central atom. ICl3 has a T-shaped molecular geometry.
tantalum → tantal
Tantalum was discovered by Anders Ekeberg (Sweden) in 1802. The origin of the name comes from the Greek word Tantalos meaning father of Niobe in Greek mythology, (tantalum is closely related to niobium in the periodic table). It is rare, grey, heavy, hard but ductile, metal with a high melting point. Exposed surfaces form corrosion resistant oxide film. Attacked by HF and fused alkalis. Metal ignites in air. Tantalum always found with niobium. Chiefly occurs in the mineral tantalite. Often used as an economical substitute for platinum. Tantalum pentoxide is used in capacitors and in camera lenses to increase refracting power. It and its alloys are corrosion and wear resistant so it is used to make surgical and dental tools.
tetrahedral molecular geometry → tetraedarska geometrija molekule
Tetrahedral is a molecular shape that results when there are four bonds and no lone pairs around the central atom in the molecule. The atoms bonded to the central atom lie at the corners of a tetrahedron with 109.5° angles between them. Molecules with an tetrahedral electron pair geometries have sp3 hybridization at the central atom. The ammonium ion (NH4+) and methane (CH4) have a tetrahedral molecular geometry.
Citing this page:
Generalic, Eni. "Elektronska konfiguracija." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table