valence shell → valentna ljuska
Valence shell is the shell corresponding to the highest value of principal quantum number in the atom. The valence electrons in this shell are on average farther from the nucleus than other electrons. They are often directly involved in chemical reaction.
vertical ionisation energy → vertikalna energija ionizacije
Vertical ionisation energy is the energy required to remove an electron from an atom, molecule, or ion in the gas phase without moving any nuclei. The vertical ionisation energy is greater than or equal to the adiabatic ionisation energy.
wrinkled filter paper → nabrani filtar-papir
Wrinkled filter paper is a filter made of wrinkled filter paper, by which, due to enlarged surface, fast filtering is achieved.
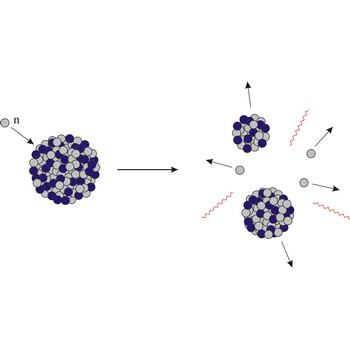
gamma radiation → gama-zračenje
Gamma radiation is electromagnetic radiation of extremely short wavelength. Gamma radiation ranges in energy from about 10-15 J to 10-10 J (10 keV to 10 MeV) (wavelength less than about 1 pm). Gamma rays are emitted by excited atomic nuclei during the process of passing to a lower excitation state.
Gamma rays are extremely penetrating and are absorbed by dense materials like lead and uranium. Exposure to gamma radiation may be lethal.
Gauss’ law for electrostatics → Gaussov zakon za elektrostatiku
Gauss’ law describes the relation between charge and electric field in static situations, so it is equivalent to Coulomb’s law, which can be derived from Gauss’ law. Gauss’ law states that the net flux of electric field, Φ, through an imaginary closed surface, S, - a Gaussian surface - is equal to the net charge, q, inside that closed surface:
where electric flux Φ through Gaussian surface is given by:
ε0 is the permittivity constant and dS is a surface element.
hassium → hassij
Hassium was discovered by Peter Armbruster, Gottfried Münzenber and their co-workers at the Heavy Ion Research Laboratory (Gesellschaft für Schwerionenforschung, GSI) in Darmstadt, Germany in 1984. The origin of the name is the Latin word Hassias meaning Hess, the German state. It is synthetic radioactive metal. Hassium was produced bythe bombardment of lead-208 with iron-58.
heavy water → teška voda
Water molecules are composed of two hydrogen atoms and one oxygen atom (H2O). If the hydrogen atoms of a water molecule are replaced by deuterium atoms, the result is heavy water (D2O). Deuterium differs from hydrogen by having one neutron in the nucleus of the atom. There is approx. one part in 5000 D2O in normal water and it can be concentrated by electrolysis. Heavy water has a higher boiling point (101.4 °C) and melts at 3.6 °C. Heavy water is 20/18=1.11 times heavier than ordinary water.
Gratzel solar cell → Gratzelova sunčeva ćelija
Grätzel solar cell is photoelectrochemical cell, developed by Michael Grätzel and collaborators, simulates some characteristics of the natural solar cell, which enables photosynthesis take place. In natural solar cell the chlorophyll molecules absorb light (most strongly in the red and blue parts of the spectrum, leaving the green light to be reflected). The absorbed energy is sufficient to knock an electron from the excited chlorophyll. In the further transport of electron, other molecules are involved, which take the electron away from chlorophyll. In Grätzel cell, the tasks of charge-carrier generation and transport are also assigned to different species.
His device consists of an array of nanometre-sized crystallites of the semiconductor titanium dioxide, welded together and coated with light-sensitive molecules that can transfer electrons to the semiconductor particles when they absorb photons. So, light-sensitive molecules play a role equivalent to chlorophyll in photosynthesis. In Grätzel cell, the light-sensitive molecule is a ruthenium ion bound to organic bipyridine molecules, which absorb light strongly in the visible range; titanium dioxide nanocrystals carry the received photoexcited electrons away from electron donors. On the other hand, a donor molecule must get back an electron, so that it can absorb another photon. So, this assembly is immersed in a liquid electrolyte containing molecular species (dissolved iodine molecules) that can pick up an electron from an electrode immersed in the solution and ferry it to the donor molecule. These cells can convert sunlight with efficiency of 10 % in direct sunlight and they are even more efficient in diffuse daylight.
histidine → histidin
Histidine is an electrically charged amino acids with basic side chains. It is an essential amino acid, which means that humans cannot synthesize it, so it must be ingested. Histidine is perhaps the most common and versatile catalytic residue in proteins. The imidazole sidechain of histidine has a pKa of approximately 6.0. This means that, at physiologically relevant pH values, relatively small shifts in pH will change its average charge. The unprotonated imidazole is nucleophilic and can serve as a general base, while the protonated form can serve as a general acid. In addition, it is often a ligand for transition metal ions such as iron and zinc.
- Abbreviations: His, H
- IUPAC name: 2-amino-3-(1H-imidazol-5-yl)propanoic acid
- Molecular formula: C6H9N3O2
- Molecular weight: 155.15 g/mol
Citing this page:
Generalic, Eni. "Efektivni naboj jezgre." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table