metre → metar
Metre (m) is the SI base unit of length.
The meter is the length of the path travelled by light in vacuum during a time interval of 1/299 792 458 s.
This definition, adopted by the General Conference on Weights and Measure in October 1983, replaced the 1967 definition based on the krypton lamp.
Moh’s scale → Mohsova skala
Mohs’ scale of mineral hardness characterises the scratch resistance of various minerals through the ability of a harder material to scratch a softer. It was created by the German mineralogist Friedrich Mohs (1773-1839). Mohs based the scale on the ten readily available minerals.
| Hardness | Mineral |
|---|---|
| 1 | talc (Mg3Si4O10(OH)2) |
| 2 | gypsum (CaSO4·2H2O) |
| 3 | calcite (CaCO3) |
| 4 | fluorite (CaF2) |
| 5 | apatite (Ca5(PO4)3(OH-,Cl-,F-)) |
| 6 | orthoclase feldspar (KAlSi3O8) |
| 7 | quartz (SiO2) |
| 8 | topaz (Al2SiO4(OH-,F-)2) |
| 9 | corundum (Al2O2) |
| 10 | diamond (C) |
monosaccharide → monosaharid
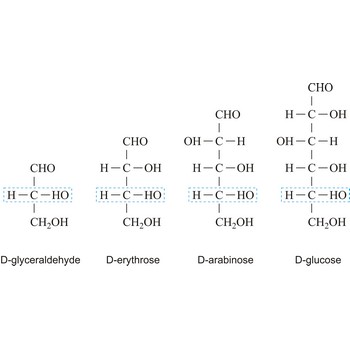
Monosaccharides are carbohydrates, with the general formula Cn(H2O)n, that cannot be decomposed to a simpler carbohydrates by hydrolysis.
Depending on whether the molecule contains an aldehyde group (-CHO) or a ketone group (-CO-) monosaccharide can be a polyhydroxy aldehyde (aldose) or a polyhydroxy ketone (ketose). These aldehyde and ketone groups confer reduction properties on monosaccharides. They are also classified according to the number of carbon atoms they contain: trioses have three carbon atoms, tetroses four, pentoses five, hexoses six, heptoses seven, etc. These two systems of classification are often combined. For example, a six-carbon polyhydroxy aldehyde such as D-glucose is an aldohexose, whereas a six-carbon polyhydroxy ketone such as D-fructose is a ketohexose.
The notations D and L are used to describe the configurations of carbohydrates. In Fischer projections of monosaccharides, the carbonyl group is always placed on top (in the case of aldoses) or as close to the top as possible (in the case of ketoses). If the OH group attached to the bottom-most asymmetric carbon (the carbon that is second from the bottom) is on the right, then the compound is a D-sugar. If the OH group is on the left, then the compound is an L-sugar. Almost all sugars found in nature are D-sugars.
Monosaccharides can exist as either straight-chain or ring-shaped molecules. During the conversion from straight-chain form to cyclic form, the carbon atom containing the carbonyl oxygen, called the anomeric carbon, becomes a chiral center with two possible configurations (anomers), α and β. When the stereochemistry of the first carbon matches the stereochemistry of the last stereogenic center the sugar is the α-anomer when they are opposite the sugar is the β-anomer.
nickel → nikal
Nickel was discovered by Axel Fredrik Cronstedt (Sweden) in 1751. The origin of the name comes from the German word kupfernickel meaning Devil’s copper or St Nicholas’s (Old Nick’s) copper. It is hard, malleable, silvery-white metal. Soluble in acids, resist alkalis. It can be polished to a lustrous finish. Resists corrosion in air under normal conditions. Nickel is chiefly found in pentlandite [(Ni,Fe)9S8] ore. The metal is produced by heating the ore in a blast furnace which replaces the sulfur with oxygen. The oxides are then treated with an acid that reacts with the iron not the nickel. Used in electroplating and metal alloys because of its resistance to corrosion. Also in nickel-cadmium batteries, as a catalyst and for coins.
noble gas → plemeniti plin
Noble gas refers to any element of the group of six elements in group 18 of the periodic table. They are helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn). Unlike most elements, the noble gases are monoatomic. The atoms have stable configurations of electrons. Therefore, under normal conditions they do not form compounds with other elements.
They were generally called inert gases until about 1962 when xenon tetrafluoride, XeF4, was produced in the laboratory. This was the first report of a stable compound of a noble gas with another single element.
Ostwald’s viscometer → Ostwaldov viskozimetar
Ostwald viscometer, also known as U-tube viscometer or capillary viscometer is a device used to measure the viscosity of the liquid with a known density. The method of determining viscosity with this instrument consists of measuring the time for a known volume of the liquid (the volume contained between the marks A and B) to flow through the capillary under the influence of gravity. Ostwald viscometers named after the German chemist Wilhelm Ostwald (1853-1932).
The instrument must first be calibrated with materials of known viscosity such as pure (deionized) water. Knowing the value of viscosity of one liquid, one can calculate the viscosity of other liquid.
where η1 and η2 are viscosity coefficients of the liquid and water, and ρ1 and ρ2 are the densities of liquid and water, respectively.
Newton’s gravitational law → Newtonov zakon gravitacije
Every object in the universe attracts every other object with a force (gravitational force FG) directed along the line through centres of the two objects that is proportional to the product of their masses and inversely proportional to the square of the distance between them.
m1 and m2 are masses of the two objects and r is the distance between them. G is universal constant of gravitation, which equals 6.67•10-26 N m2 kg-2. Strictly speaking, this law applies only to objects that can be considered pointlike object. Otherwise, the force has to be found by integrating the forces between various mass elements.
It is more properly to express Newton’s gravitational law by vector equation:
in which r1 and r2 are position vectors of masses m1 and m2.
Gravitational forces act on distance. Newton’s gravitational law is derived from Kepler’s law for planetary motion, using a physical assumption considering Sun as the centre and the source of gravitational force.
Additionally, every object moves in the direction of the force acting on it, with acceleration that is inversely proportional to the mass of object. For bodies on the surface of Earth, the distance r in gravitational law formula is practically equal to the Earth radius, RE. If the mass of the body on Earth surface is m and the mass of earth is ME, the gravitational force acting on that body can be expressed as:
where g is gravitational acceleration which is, although dependent on geographical latitude, usually considered as constant equal to 9.81 m s-2.
oxo compound → okso-spoj
Oxo compounds are organic compounds that contain the karbonyl group, C=O. The term thus embraces aldehydes, carboxylic acids, ketones, amides, and esters.
Citing this page:
Generalic, Eni. "Alicikli�ki spojevi." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table