periodic table of the elements → periodni sustav elemenata
Periodic table is a table of elements, written in sequence in the order of atomic number or atomic weight and arranged in horizontal rows (periods) and vertical columns (groups) to illustrate the occurrence of similarities in the properties of the elements as a periodic function of the sequence. The original form was proposed by Dmitri Mendeleev (1834-1907) in 1869, using relative atomic masses.
glass → staklo
Glass is a brittle transparent solid with the molecular structure of a liquid. It is made by fusing together sand (SiO2), soda (Na2CO3), and lime (CaCO3) with small quantities other compounds. It is used for window panes and mirrors, for articles of table and culinary use, for lenses, and various articles of ornament.
actinides → aktinoidi
Actinides (actinons or actinoids) are the fourteen elements from thorium to lawrencium inclusive, which follow actinium in the periodic table. The position of actinium is somewhat equivocal and, although not itself an actinide, it is often included with them for comparative purpose. The series includes the following elements: thorium (Th), protactinium (Pa), uranium (U), neptunium (Np), plutonium (Pu), amercium (Am), curium (Cm), berkelium (Bk), californium (Cf), einsteinium (Es), fermium (Fm), mendelevium (Md), nobelium (No) and lawrencium (Lr). Every known isotope of the actinide elements is radioactive. Traces of Pa, Np and Pu are consequently found, but only Th and U occur naturally to any useful extent.
alkali metal → alkalijski metal
Alkali metal is a term that refers to six elements: lithium (Li), sodium (Na), potassium (K), rubidium (Rb), caesium (Cs), and francium (Fr). These elements make up group 1 of the periodic table of elements. They all form singly charged positive ions, and are extremely reactive. They react violently with water, forming hydroxides and releasing hydrogen gas and heat. Caesium and francium are the most reactive and lithium is the least.
alkaline earth metal → zemnoalkalijski metal
Alkali earth metal is a term that refers to six elements: beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra). These elements make up group 2 of the periodic table of elements. They all exhibit a single oxidation state, +2. They are all light and very reactive. Barium and radium are the most reactive and beryllium is the least.
To denote slightly soluble metal oxides chemists formerly used the term "earth". The oxides of barium, strontium, and calcium resemble alumina (Al2O3), a typical "earth", but form alkaline mixtures with water. For this reason barium, strontium, and calcium were called alkaline earth metals. This name has now been extended to include all of the elements of group 2.
Bravais lattice → Bravaisova rešetka
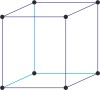
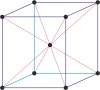
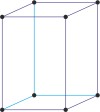
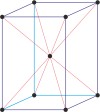
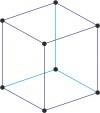
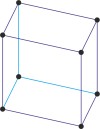
Bravais lattice is a set of points constructed by translating a single point in discrete steps by a set of basis vectors. The French crystallographer Auguste Bravais (1811-1863) established that in three-dimensional space only fourteen different lattices may be constructed. All crystalline materials recognised till now fit in one of these arrangements. The fourteen three-dimensional lattices, classified by crystal system, are shown to the bottom.
|
Crystal system
|
Bravais lattices
|
|||
|
cubic a=b=c α=β=γ=90° |
 |
 |
 |
|
|
|
simple cubic
|
body-centered cubic
|
face-centered cubic
|
|
|
tetragonal a=b≠c α=β=γ=90° |
 |
 |
||
|
|
simple tetragonal
|
body-centered tetragonal
|
||
|
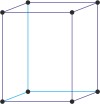
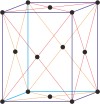
orthorhombic a≠b≠c α=β=γ=90° |
 |
 |
 |
 |
|
|
simple orthorhombic
|
base-centered orthorhombic
|
body-centered orthorhombic
|
face-centered orthorhombic
|
|
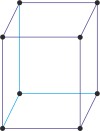
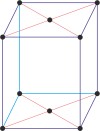
monoclinic a≠b≠c α=γ=90°≠β |
 |
 |
||
|
|
simple monoclinic
|
base-centered monoclinic
|
||
|
hexagonal a=b≠c α=β=90° γ=120° |
 |
|||
|
|
hexagonal
|
|||
|
rhombohedral a=b=c α=β=γ≠90° |
 |
|||
|
|
rhombohedral
|
|||
|
triclinic a≠b≠c α≠β≠γ≠90° |
 |
|||
|
triclinic
|
||||
invertase → invertaza
Invertase (sucrase, saccharase, beta-fructofuranosidase) is an enzyme present in yeast and in the intestinal juice of animals that catalyze the hydrolysis of table sugar (sucrose, saccharose) to the simple sugars, glucose and fructose. This equimolar mixture of glucose and fructose is called invert sugar.
calomel electrode → kalomel elektroda
Calomel electrode is a type of half cell in which the electrode is mercury coated with calomel (Hg2Cl2) and the electrolyte is a solution of potassium chloride and saturated calomel. In the calomel half cell the overall reaction is
Table: Dependence of potential of calomel electrode upon temperature and concentration of KCl according to standard hydrogen electrode
| Potential vs. SHE / V | |||
|---|---|---|---|
| t / °C | 0.1 mol dm-3 | 3.5 mol dm-3 | sat. solution |
| 15 | 0.3362 | 0.254 | 0.2511 |
| 20 | 0.3359 | 0.252 | 0.2479 |
| 25 | 0.3356 | 0.250 | 0.2444 |
| 30 | 0.3351 | 0.248 | 0.2411 |
| 35 | 0.3344 | 0.246 | 0.2376 |
Citing this page:
Generalic, Eni. "Panel tabi." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table


