Heisenberg uncertainty principle → Heisenbergov princip neodređenosti
Heisenberg uncertainty principle is the principle that it is not possible to know with unlimited accuracy both the position and momentum of a particle. The German physicist Werner Heisenberg (1901-1976) discovered this principle in 1927.
Le Chatelier’s principle → Le Chatelierov princip
The idea that a system at equilibrium will respond to a stress placed upon it in such a manner as to partially offset that stress. The principle was first stated in 1888 by the French physical chemist Henri Le Chatelier (1850-1936).
Pauli exclusion principle → Paulijev princip zabrane
Pauli exclusion principle is the statement that two electrons in an atom cannot have identical all four quantum numbers. It was first formulated in 1925 by the Austrian-born Swiss physicst Wolfgang Ernst Pauli (1900-1958).
analytical balance → analitička vaga
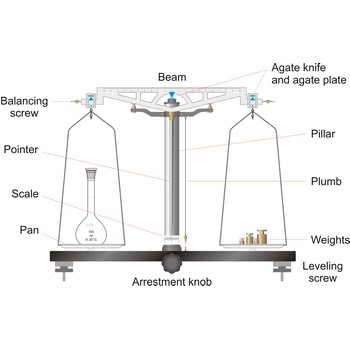
Analytical balances are instruments used for precise determining mass of matter. Analytical balances are sensitive and expensive instruments, and upon their accuracy and precision the accuracy of analysis result depends. The most widely used type of analytical balances are balances with a capacity of 100 g and a sensitivity of 0.1 mg. Not one quantitative chemical analysis is possible without usage of balances, because, regardless of which analytical method is being used, there is always a need for weighing a sample for analysis and the necessary quantity of reagents for solution preparation.
The working part of the balance is enclosed in a glass-fitted case. The baseplate is usually of black glass or black slate. The beam has agate knife-edges at its extremes, supporting stirrups from which balance pans are suspended. Another agate or steel knife-edge is fixed exactly in the middle of the beam on its bottom side. This knife-edge faces downwards and supports the beam. When not in use and during loading or unloading of the pans, the balance should be arrested.
The principle of operation of a modern laboratory balance bears some resemblance to its predecessor - the equal arm balance. The older instrument opposed the torque exerted by an unknown mass on one side of a pivot to that of an adjustable known weight on the other side. When the pointer returned to the center position, the torques must be equal, and the weight was determined by the position of the moving weights.
Modern electronic laboratory balances work on the principle of magnetic force restoration. In this system, the force exerted by the object being weighed is lifted by an electromagnet. A detector measures the current required to oppose the downward motion of the weight in the magnetic field.
biochemistry → biokemija
Biochemistry is the study of the chemistry of living organisms, especially the structure and function of their chemical components (principally proteins, carbohydrates, lipids and nucleic acids).
Bohr atom → Bohrov atom
Bohr atom is a model of the atom that explains emission and absorption of radiation as transitions between stationary electronic states in which the electron orbits the nucleus at a definite distance. The Bohr model violates the Heisenberg uncertainty principle since it postulates definite paths and moment for electrons as they move around the nucleus. Modern theories usually use atomic orbitals to describe the behaviour of electrons in atoms.
balance → vaga
Balance is an instrument to measure the mass (or weight) of a body. Balance beam type scales are the oldest type and measure weight using a fulcrum or pivot and a lever with the unknown weight placed on one end of the lever, and a counterweight applied to the other end. When the lever is balanced, the unknown weight and the counterweight are equal. The equal-arm balance consists of two identical pans hung from either end of a centrally suspended beam. The unequal-arm balance is made with one arm of the balance much longer than the other.
More modern substitution balances use the substitution principle. In this calibrated weights are removed from the single lever arm to bring the single pan suspended from it into equilibrium with a fixed counter weight. The substitution balance is more accurate than the two-pan device and enables weighing to be carried out more rapidly.
Electromagnetic force restoration balances also use a lever system but a magnetic field is used to generate the force on the opposite end of the lever and balance out the unknown mass. The current used to drive the magnetic coil is proportional to the mass of the object placed on the platform.
Carnot cycle → Carnotov kružni proces
Carnot cycle is the most efficient cycle of operations for a reversible heat engine. Published in 1824 by French physicist Nicolas Léonard Sadi Carnot (1796-1832), it consists of four operations on the working substance in the engine:
1-2: Isothermal expansion at thermodynamic temperature T1 with heat QH taken in.
2-3: Adiabatic expansion with a fall of temperature to T2.
3-4: Isothermal compression at temperature T2 with heat QC given out.
4-1: Adiabatic compression at temperature back to T1.
According to the Carnot principle, the efficiency of any reversible heat engine depends only on the temperature range through which it works, rather than the properties of the working substances.
radiation → radijacija
The process of heat transfer which occurs through empty space and can also occur in matter, in the form of electro-magnetic (EM) waves, is called radiation or radiant heat. Whenever EM radiation is emitted and then absorbed, heat is transferred. This principle is used in microwave ovens, laser cutting, and RF hair removal.
Citing this page:
Generalic, Eni. "Le Chatelierov princip." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table