relative molecular mass → relativna molekularna masa
Relative molecular mass (Mr) is the ratio of the average mass per molecule or specified entity of a substance to 1/12 of the mass of nuclide 12C. Also called molecular weight. It is equal to the sum of the relative atomic masses of all the atoms that comprise a molecule. For example
Mr(H2SO4) = 2·Ar(H) + Ar(S) + 4·Ar(O)
= 2·1.0079 + 32.066 + 4·15.999
= 2.0158 + 32.066 + 63.996
= 98.078
rhenium → renij
Rhenium was discovered by Walter Noddack, Ida Tacke and Otto Berg (Germany) in 1925. The origin of the name comes from the Latin word Rhenus meaning river Rhine. It is rare and costly, dense, silvery-white metal. Tarnishes in moist air. Resists corrosion and oxidation. Dissolves in nitric and sulfuric acids. Has a very high melting point. Rhenium is found in small amounts in gadolinite and molybdenite. Mixed with tungsten or platinum to make filaments for mass spectrographs. Its main value is as a trace alloying agent for hardening metal components that are subjected to continuous frictional forces.
rhodium → rodij
Rhodium was discovered by William Hyde Wollaston (England) in 1804. The origin of the name comes from the Greek word rhodon meaning rose. It is hard, silvery-white metal. Inert in air and acids. Reacts with fused alkalis. Rhodium is obtained as a by-product of nickel production. Used as a coating to prevent wear on high quality science equipment and with platinum to make thermocouples.
ruthenium → rutenij
Ruthenium was discovered by Karl Karlovich Klaus (Russia) in 1844. The origin of the name comes from the Latin word Ruthenia meaning Russia. It is rare, extremely brittle, silver-grey metal. Unaffected by air, water or acids. Reacts with very hot (molten) alkalis. Ruthenium is found in pentlandite and pyroxinite. Used to harden platinum and palladium. Aircraft magnetos use platinum alloy with 10 % ruthenium.
sedimentary rocks → sedimentne stijene
Sedimentary Rocks are formed by the accumulation and subsequent consolidation of sediments into various types of rock. There are three major types of sedimentary rocks:
Biogenic sedimentary rocks are formed from organic processes when organisms use materials dissolved in water to build a shell or other skeletal structure.
Clastic sedimentary rocks are composed directly of the sediments or fragments from other rocks.
Chemical sedimentary rocks are formed through evaporation of a chemical rich solution.
Based on their sizes, sediment particles are classified, based on their size, into six general categories:
- boulder (>256 mm)
- cobble (64 - 256 mm)
- gravel (2 - 64 mm)
- sand (1/16 - 2 mm)
- silt (1/256 - 1/16 mm)
- clay (<1/256 mm)
selenium → selenij
Selenium was discovered by Jöns Jakob Berzelius (Sweden) in 1817. The origin of the name comes from the Greek word selene meaning moon. It is soft metalloid similar to sulfur. Ranges from grey metallic to red glassy appearance. Unaffected by water. Soluble in alkalis and nitric acid. Burns in air. Toxic by inhalation or ingestion. Selenium is obtained from lead, copper and nickel refining. Conducts electricity when struck by light. Light causes it to conduct electricity more easily. It is used in photoelectric cells, TV cameras, xerography machines and as a semiconductor in solar batteries and rectifiers. Also colours glass red.
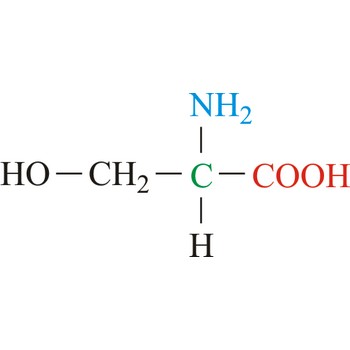
serine → serin
Serine is neutral amino acids with polar side chains. It is one of two hydroxyl amino acids. Both are commonly considered to by hydrophilic due to the hydrogen bonding capacity of the hydroxyl group. Serine often serves as a nucleophile in many enzyme active sites, and is best known for its role in the serine proteases. Serine is a site of phosphorylation and glycosylation which is important for enzyme regulation and cell signaling. It is not essential to the human diet, since it is synthesized in the body from other metabolites, including glycine.
- Abbreviations: Ser, S
- IUPAC name: 2-amino-3-hydroxypropanoic acid
- Molecular formula: C3H7NO3
- Molecular weight: 105.09 g/mol
silicon → silicij
Silicon was discovered by Jöns Jacob Berzelius (Sweden) in 1824. The origin of the name comes from the Latin word silicis meaning flint. Amorphous form of silicon is brown powder; crystalline form has grey metallic appearance. Solid form unreactive with oxygen, water and most acids. Dissolves in hot alkali. Silica dust is a moderately toxic acute irritant. Silicon makes up major portion of clay, granite, quartz and sand. Commercial production depends on a reaction between sand (SiO2) and carbon at a temperature of around 2200 °C. Used in glass as silicon dioxide (SiO2). Silicon carbide (SiC) is one of the hardest substances known and used in polishing. Also the crystalline form is used in semiconductors.
silver → srebro
Silver has been known since ancient times. The origin of the name comes from the Latin word argentum meaning silver. It is silvery-ductile and malleable metal. Stable in water and oxygen. Reacts with sulfur compounds to form black sulfides. Silver is found in ores called argentite (AgS), light ruby silver (Ag3AsS3), dark ruby silver (Ag3SbS3) and brittle silver. Used in alloys for jewellery and in other compounds for photography. It is also a good conductor, but expensive.
sulfur → sumpor
Sulfur has been known since ancient times. The origin of the name comes from the Sanskrit word sulvere meaning sulphur; also from the Latin word sulphurium meaning sulphur. It is pale yellow, odourless, brittle solid, which is insoluble in water but soluble in carbon disulfide. Sulfur is found in pure form and in ores like cinnabar, galena, sphalerite and stibnite. Pure form is obtained from underground deposits by the Frasch process. Used in matches, gunpowder, medicines, rubber and pesticides, dyes and insecticides. Also for making sulfuric acid (H2SO4).
Citing this page:
Generalic, Eni. "Zasićena masna kiselina." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table