resistance → električni otpor
Electrical resistance (R) of a given object is the opposition to the passage of an electric current through that object. The SI unit of electrical resistance is the ohm, represented by the Greek letter omega (Ω). Resistance is the electric potential difference divided by the current when there is no electromotive force in the conductor. This definition applies to direct current. For a conductor of uniform cross section with area A and length L, and whose resistivity is ρ, the resistance is given by
resistivity → električna otpornost
Electrical resistivity, or specific resistance (ρ) is the electric field strength divided by the current density when there is no electromotive force in the conductor. Resistivity is an intrinsic property of a material. Materials with low resistivity are good conductors of electricity and materials with high resistivity are good insulators.
For a conductor of uniform cross section with area A and length L, and whose resistance is R, the resistivity is given by
The SI unit is Ω m.
reversible process → reverzibilan proces
Reversible process or reaction is those that can be reversed by an infinitesimally small change in conditions. For example, ice and water coexist at 101 325 Pa and 0 °C; a very slight temperature increase causes the ice to melt; a tiny temperature decrease causes the water to freeze. Melting or freezing under these conditions can be considered reversible.
rhenium → renij
Rhenium was discovered by Walter Noddack, Ida Tacke and Otto Berg (Germany) in 1925. The origin of the name comes from the Latin word Rhenus meaning river Rhine. It is rare and costly, dense, silvery-white metal. Tarnishes in moist air. Resists corrosion and oxidation. Dissolves in nitric and sulfuric acids. Has a very high melting point. Rhenium is found in small amounts in gadolinite and molybdenite. Mixed with tungsten or platinum to make filaments for mass spectrographs. Its main value is as a trace alloying agent for hardening metal components that are subjected to continuous frictional forces.
rubidium → rubidij
Rubidium was discovered by Robert Bunsen and Gustav Kirchhoff (Germany) in 1861. The origin of the name comes from the Latin word rubidius meaning dark red or deepest red. It is soft, silvery-white, highly reactive metal. Ignites in air. Reacts violently with water or oxidants. Rubidium occurs abundantly, but so widespread that production is limited. Usually obtained from lithium production. Used as a catalyst, photocells and vacuum and cathode-ray tubes.
ruthenium → rutenij
Ruthenium was discovered by Karl Karlovich Klaus (Russia) in 1844. The origin of the name comes from the Latin word Ruthenia meaning Russia. It is rare, extremely brittle, silver-grey metal. Unaffected by air, water or acids. Reacts with very hot (molten) alkalis. Ruthenium is found in pentlandite and pyroxinite. Used to harden platinum and palladium. Aircraft magnetos use platinum alloy with 10 % ruthenium.
salinity → salinitet
Salinity (S) is a measure of the quantity of dissolved salts in seawater. It is formally defined as the total amount of dissolved solids in seawater in parts per thousand (‰) by weight when all the carbonate has been converted to oxide, the bromide and iodide to chloride, and all organic matter is completely oxidized.
Chlorinity is the oldest of the salinity measures considered and is still a corner-stone in the study of dissolved material in seawater. Based on the principle of constant relative proportions it provides a measure of the total amount of dissolved material in seawater in terms of the concentration of halides. The relationship between chlorinity (Cl) and salinity as set forth in Knudsen’s tables is
In 1962, however, a better expression for the relationship between total dissolved salts and chlorinity was found to be
Practical Salinity (SP) was introduced as a replacement for Chlorinity. Practical Salinity is is relatively easy to measure using standard conductometers, measurements are more precise and less time consuming than measurements of Chlorinity and accurate measurements can even be made in situ. Practical salinity SP is defined on the Practical Salinity Scale of 1978 (PSS-78) in terms of the conductivity ratio K15 which is the electrical conductivity of the sample at temperature t68 = 15 °C and pressure equal to one standard atmosphere, divided by the conductivity of a standard potassium chloride (KCl) solution at the same temperature and pressure. The mass fraction of KCl in the standard solution is 0.0324356 (32.4356 g of KCl in 1 kg of solution).
Note that Practical Salinity is a unit-less quantity. Though sometimes convenient, it is technically incorrect to quote Practical Salinity in "psu". For most purposes one can assume that the psu and the ‰, are synonymous.
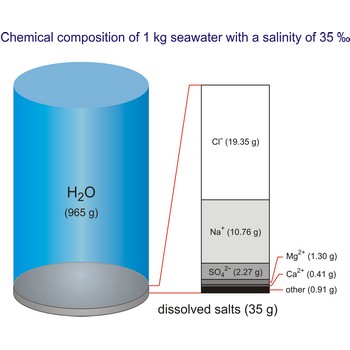
The global average salinity of ocean waters is about 35 ‰, that is, about 35 g of solid substances are dissolved in 1 kg of seawater.
saturated fatty acid → zasićena masna kiselina
Saturated fatty acid is a fatty acid carrying the maximum possible number of hydrogen atoms (It doesn’t have any double bounds in the alkyl chain). The most important of these are:
| Butyric (butanoic acid) | CH3(CH2)2COOH |
| Lauric (dodecanoic acid) | CH3(CH2)10COOH |
| Myristic (tetradecanoic acid) | CH3(CH2)12COOH |
| Palmitic (hexadecanoic acid) | CH3(CH2)14COOH |
| Stearic (octadecanoic acid) | CH3(CH2)16COOH |
| Arachidic (eicosanoic acid) | CH3(CH2)18COOH |
scandium → skandij
Scandium was discovered by Lars Fredrik Nilson (Sweden) in 1879. The origin of the name comes from the Latin word Scandia meaning Scandinavia. It is fairly soft, silvery-white metal. Burns easily. Tarnishes readily in air. Reaction with water releases hydrogen. Reacts with air and halogens. Scandium occurs mainly in the minerals thortveitile (~34 % scandium) and wiikite. Also in some tin and tungsten ores. Pure scandium is obtained as a by-product of uranium refining. Scandium metal is used in some aerospace applications. Scandium oxide (Sc2O2) is used in the manufacture of high-intensity electric lamps. Scandium iodide (ScI3) is used in lamps that produce light having a colour closely matching natural sunlight.
seawater → more
Seawater is a complex mixture of 96.5 % water, 3.5 % salts, and smaller amounts of other substances, including dissolved inorganic and organic materials, particulates, and a few atmospheric gases. The world's oceans cover nearly 71 % (361 840 000 km2) of the Earth's surface (510 100 000 km2), with an average depth of 3 682.2 m.
The density of seawater is higher than that of fresh water because of its higher salinity. Seawater's freezing point is lower than that of pure water and its boiling point is higher. The average salinity of the ocean is 35 ‰, which means that for every kilograms of water, there are 35 g of salt. The relative abundance of the major salts in seawater are constant regardless of the ocean. Only six elements and compounds comprise about 99 % of sea salts: chlorine (Cl-), sodium (Na+), sulfur (SO42-), magnesium (Mg2+), calcium (Ca2+), and potassium (K+).
Citing this page:
Generalic, Eni. "Voda." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table