boiling point → vrelište
Boiling point is the temperature at which the vapour pressure of a liquid is equal to the external pressure on the liquid. The standard boiling point is the temperature at which the vapour pressure of a liquid equals standard pressure (101 325 Pa).
angular momentum → moment količine gibanja
Angular momentum is a physical quantity defined for rotating motion (in analogy to momentum that is defined for linear motion). If a body rotates around a specified axis, its angular momentum equals
Where I is the rotational inertia concerning that axis and ω is the angular velocity of the body.
Angular momentum can also be defined for a point-like body concerning a specified origin (in that case, it is not necessary that the point-like body undergoes circular motion). Rotational inertia of the point-like body, concerning that origin equals:
Where m is the mass of the body and r is its distance from the origin.
angular velocity → kutna brzina
A point-like object that undergoes circular motion changes its angular position from initial Θi to final Θf, relative to a fixed axis, specified in a coordinate system with an origin that coincides the centre of the circular path of object. The change in its angular position is called angular displacement ΔΘ = Θf - Θi. Also, a rigid body that rotates about a specified rotation axis, changing its angular position from initial Θi to final Θf, undergoes an angular displacement ΔΘ.
The average angular velocity, ωav, is the ratio of the angular displacement and the time interval Δt=tf-ti, in which that displacement occurs.
Θf and Θi are the initial and final angular position, respectively.
The instantaneous angular velocity ω is the limit of the average angular velocity, as Δt is made to approach zero.
ωav and ω are positive for the counterclockwise rotation (in direction of increasing Θ) and negative for the clockwise rotation (in direction of decreasing Θ).
SI unit for angular velocity is s-1.The measure for the angle Θ is radian. The relationship between radians and degrees is:
For example, the angular velocity of the minute hand of a clock is:
Bravais lattice → Bravaisova rešetka
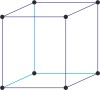
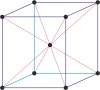
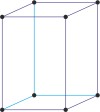
Bravais lattice is a set of points constructed by translating a single point in discrete steps by a set of basis vectors. The French crystallographer Auguste Bravais (1811-1863) established that in three-dimensional space only fourteen different lattices may be constructed. All crystalline materials recognised till now fit in one of these arrangements. The fourteen three-dimensional lattices, classified by crystal system, are shown to the bottom.
|
Crystal system
|
Bravais lattices
|
|||
|
cubic a=b=c α=β=γ=90° |
 |
 |
 |
|
|
|
simple cubic
|
body-centered cubic
|
face-centered cubic
|
|
|
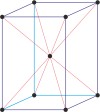
tetragonal a=b≠c α=β=γ=90° |
 |
 |
||
|
|
simple tetragonal
|
body-centered tetragonal
|
||
|
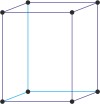
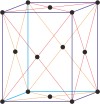
orthorhombic a≠b≠c α=β=γ=90° |
 |
 |
 |
 |
|
|
simple orthorhombic
|
base-centered orthorhombic
|
body-centered orthorhombic
|
face-centered orthorhombic
|
|
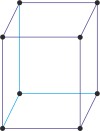
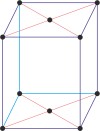
monoclinic a≠b≠c α=γ=90°≠β |
 |
 |
||
|
|
simple monoclinic
|
base-centered monoclinic
|
||
|
hexagonal a=b≠c α=β=90° γ=120° |
 |
|||
|
|
hexagonal
|
|||
|
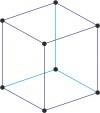
rhombohedral a=b=c α=β=γ≠90° |
 |
|||
|
|
rhombohedral
|
|||
|
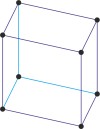
triclinic a≠b≠c α≠β≠γ≠90° |
 |
|||
|
triclinic
|
||||
Celsius temperature scale → Celsiusova temperaturna skala
For value of zero in Celsius temperature scale the freezing point of water at a pressure of 101 325 Pa is taken. The boiling point of water at a pressure of 101 325 Pa is taken as another reference point. This range is divided into 100 equal parts, and each part is an equivalent to 1 °C. Units of Celsius temperature scale (°C) and thermodynamic temperature scale (K) are identical
1 °C = 1 K.
conductometry → konduktometrija
Conductometry is a volumetric analytic method in which the end of titration (equivalent point) is defined by an electric conductivity appliance.
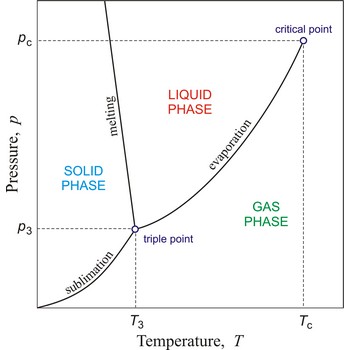
critical pressure → kritični tlak
Critical pressure is the pressure of a fluid in its critical point; i.e. when it is at its critical temperature and critical volume.
cryogenic fractionation → kriogena frakcinacija
Cryogenic fractionation is a process of separation of gases by cooling them until they enter their liquid state. Large scale gas production companies use this method to produce liquid oxygen, liquid nitrogen etc. Gases have different boiling points (the temperature at which they change from liquid to gas). Oxygen has a boiling point of -183 °C, and nitrogen a boiling point of -195.8 °C. Therefore by cooling the gas mixture to -183 °C, the oxygen can be collected as liquid and the nitrogen remains its gaseous form.
eutectic → eutektik
Eutectic is a solid solution consisting of two or more substances and having the lowest freezing point of any possible mixture of these components.
Eutectic point is the lowest temperature at which the eutectic mixture can exist in a liquid phase. A liquid having the eutectic composition will freeze at a single temperature without a change of composition.
Citing this page:
Generalic, Eni. "Trojna točka." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table