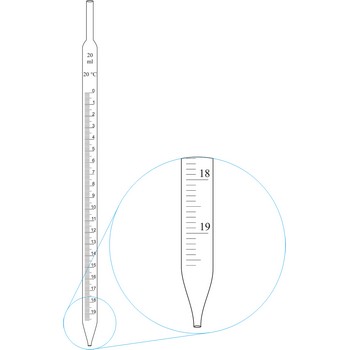
graduated pipette → graduirana pipeta
Graduated pipettes (Mohr pipette) have a scale divided into units of one and of 1/10th of a millilitre. Because of their wide necks it is less accurate than the volumetric pipette. They are used when taking volume of solutions in which accuracy does not have to be very high. By sucking in (with mouth, propipette or a water pump) the liquid is pulled in a little bit above the mark and the opening of the pipet is closed with a forefingertip. Outer wall of pipet is wiped and, with a slight forefinger loosening, the liquid is released until it reaches the mark 0. A pipette is emptied out by lifting the forefinger off and letting the liquid flow out of the pipette freely.
polar solvent → polarno otapalo
Polar solvent is a liquid with polar molecules which dissolves polar compounds, because the charges on the endings of the solvent’s molecules attract the elements from ion crystals.
Raman effect → Ramanov efekt
Raman effect is a type of scattering of electromagnetic radiation in which light suffers a change in frequency and a change in phase as it passes through a material medium. Named according to the Indian physicist C. V. Raman (1889-1970). The intensity of Raman scattering is about one-thousandth of that in Rayleigh scattering in liquids.
Gratzel solar cell → Gratzelova sunčeva ćelija
Grätzel solar cell is photoelectrochemical cell, developed by Michael Grätzel and collaborators, simulates some characteristics of the natural solar cell, which enables photosynthesis take place. In natural solar cell the chlorophyll molecules absorb light (most strongly in the red and blue parts of the spectrum, leaving the green light to be reflected). The absorbed energy is sufficient to knock an electron from the excited chlorophyll. In the further transport of electron, other molecules are involved, which take the electron away from chlorophyll. In Grätzel cell, the tasks of charge-carrier generation and transport are also assigned to different species.
His device consists of an array of nanometre-sized crystallites of the semiconductor titanium dioxide, welded together and coated with light-sensitive molecules that can transfer electrons to the semiconductor particles when they absorb photons. So, light-sensitive molecules play a role equivalent to chlorophyll in photosynthesis. In Grätzel cell, the light-sensitive molecule is a ruthenium ion bound to organic bipyridine molecules, which absorb light strongly in the visible range; titanium dioxide nanocrystals carry the received photoexcited electrons away from electron donors. On the other hand, a donor molecule must get back an electron, so that it can absorb another photon. So, this assembly is immersed in a liquid electrolyte containing molecular species (dissolved iodine molecules) that can pick up an electron from an electrode immersed in the solution and ferry it to the donor molecule. These cells can convert sunlight with efficiency of 10 % in direct sunlight and they are even more efficient in diffuse daylight.
regeneration → regeneracija
Regeneration is the process of restoring an ion exchange medium to a usable state after exhaustion. The cation exchanger is normally regenerated with hydrochloric acid and the anion exchanger with sodium hydroxide.
Henry’s law → Henryjev zakon
Henry’s law was discovered in 1801 by the British chemist William Henry (1775-1836). At a constant temperature the mass of gas dissolved in a liquid at equilibrium is proportional to the partial pressure of the gas. It applies only to gases that do not react with the solvent.
where pi is the partial pressure of component i above the solution, xi is its mole fraction in the solution, and Kx is the Henry’s law constant (a characteristic of the given gas and solvent, as well as the temperature).
Hesse’s law → Hessov zakon
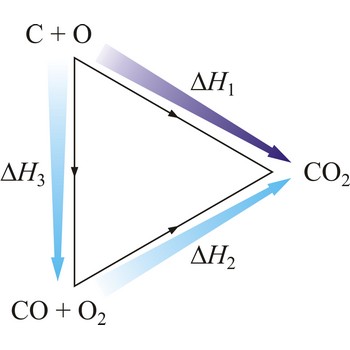
Hesse’s law says that reaction heat of some chemical change does not depend on the way in which the reaction is conducted, but only on starting and ending system state. Hesse’s law is also known as the law of constant heat summation. Hesse’s law is also known as the law of constant heat summation. The law was first put forward in 1840 by the Swiss-born Russian chemist Germain Henri Hess (1802-1850).
Hesse’s law can be used to obtain thermodynamic data that cannot be measured directly. For example, it is very difficult to control the oxidation of graphite to give pure CO. However, enthalpy for the oxidation of graphite to CO2 can easily be measured. So can the enthalpy of oxidation of CO to CO2. The application of Hess’s law enables us to estimate the enthalpy of formation of CO.
| C(s) + O2(g) →← CO2(g) | ΔrH1 = -393 kJ mol-1 |
| CO(g) + 1/2O2(g) →← CO2(g) | ΔrH2 = -283 kJ mol-1 |
| C(s) + 1/2O2(g) →← CO(g) | ΔrH3 = -110 kJ mol-1 |
The equation shows the standard enthalpy of formation of CO to be -110 kJ/mol.
Citing this page:
Generalic, Eni. "Tekuće agregatno stanje." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table