monosaccharide → monosaharid
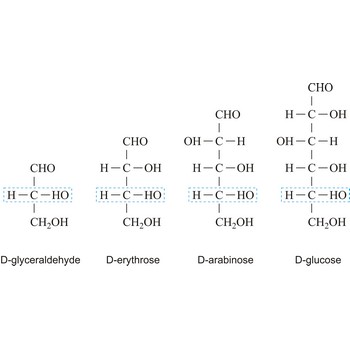
Monosaccharides are carbohydrates, with the general formula Cn(H2O)n, that cannot be decomposed to a simpler carbohydrates by hydrolysis.
Depending on whether the molecule contains an aldehyde group (-CHO) or a ketone group (-CO-) monosaccharide can be a polyhydroxy aldehyde (aldose) or a polyhydroxy ketone (ketose). These aldehyde and ketone groups confer reduction properties on monosaccharides. They are also classified according to the number of carbon atoms they contain: trioses have three carbon atoms, tetroses four, pentoses five, hexoses six, heptoses seven, etc. These two systems of classification are often combined. For example, a six-carbon polyhydroxy aldehyde such as D-glucose is an aldohexose, whereas a six-carbon polyhydroxy ketone such as D-fructose is a ketohexose.
The notations D and L are used to describe the configurations of carbohydrates. In Fischer projections of monosaccharides, the carbonyl group is always placed on top (in the case of aldoses) or as close to the top as possible (in the case of ketoses). If the OH group attached to the bottom-most asymmetric carbon (the carbon that is second from the bottom) is on the right, then the compound is a D-sugar. If the OH group is on the left, then the compound is an L-sugar. Almost all sugars found in nature are D-sugars.
Monosaccharides can exist as either straight-chain or ring-shaped molecules. During the conversion from straight-chain form to cyclic form, the carbon atom containing the carbonyl oxygen, called the anomeric carbon, becomes a chiral center with two possible configurations (anomers), α and β. When the stereochemistry of the first carbon matches the stereochemistry of the last stereogenic center the sugar is the α-anomer when they are opposite the sugar is the β-anomer.
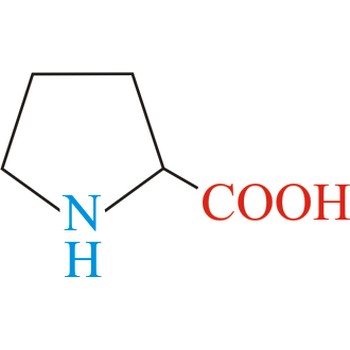
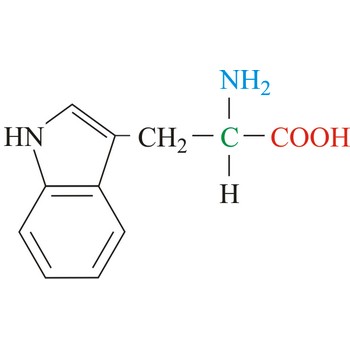
proline → prolin
Proline has an aliphatic side chain with a distinctive cyclic structure. It is unusual because it is conformationally restricted. The secondary amino (imino) group of proline residues is held in a rigid conformation that reduces the structural flexibility of polypeptide regions containing proline. It is not an essential amino acid, which means that the human body can synthesize it.
- Abbreviations: Pro, P
- IUPAC name: pyrrolidine-2-carboxylic acid
- Molecular formula: C5H9NO2
- Molecular weight: 115.13 g/mol
Newton’s gravitational law → Newtonov zakon gravitacije
Every object in the universe attracts every other object with a force (gravitational force FG) directed along the line through centres of the two objects that is proportional to the product of their masses and inversely proportional to the square of the distance between them.
m1 and m2 are masses of the two objects and r is the distance between them. G is universal constant of gravitation, which equals 6.67•10-26 N m2 kg-2. Strictly speaking, this law applies only to objects that can be considered pointlike object. Otherwise, the force has to be found by integrating the forces between various mass elements.
It is more properly to express Newton’s gravitational law by vector equation:
in which r1 and r2 are position vectors of masses m1 and m2.
Gravitational forces act on distance. Newton’s gravitational law is derived from Kepler’s law for planetary motion, using a physical assumption considering Sun as the centre and the source of gravitational force.
Additionally, every object moves in the direction of the force acting on it, with acceleration that is inversely proportional to the mass of object. For bodies on the surface of Earth, the distance r in gravitational law formula is practically equal to the Earth radius, RE. If the mass of the body on Earth surface is m and the mass of earth is ME, the gravitational force acting on that body can be expressed as:
where g is gravitational acceleration which is, although dependent on geographical latitude, usually considered as constant equal to 9.81 m s-2.
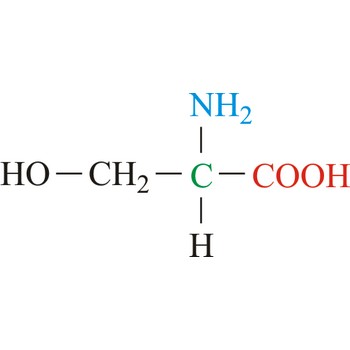
serine → serin
Serine is neutral amino acids with polar side chains. It is one of two hydroxyl amino acids. Both are commonly considered to by hydrophilic due to the hydrogen bonding capacity of the hydroxyl group. Serine often serves as a nucleophile in many enzyme active sites, and is best known for its role in the serine proteases. Serine is a site of phosphorylation and glycosylation which is important for enzyme regulation and cell signaling. It is not essential to the human diet, since it is synthesized in the body from other metabolites, including glycine.
- Abbreviations: Ser, S
- IUPAC name: 2-amino-3-hydroxypropanoic acid
- Molecular formula: C3H7NO3
- Molecular weight: 105.09 g/mol
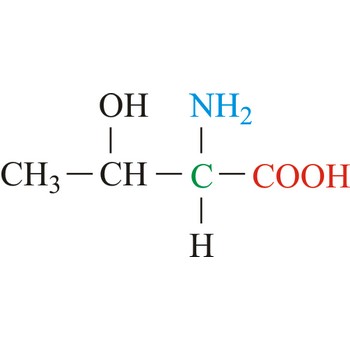
threonine → treonin
Threonine is neutral amino acids with polar side chains. It differs from serine by having a methyl substituent in place of one of the hydrogens on the β carbon. Threonine is a site of phosphorylation and glycosylation which is important for enzyme regulation and cell signaling. It is an essential amino acid, which means that humans cannot synthesize it, so it must be ingested.
- Abbreviations: Thr, T
- IUPAC name: 2-amino-3-hydroxybutanoic acid
- Molecular formula: C4H9NO3
- Molecular weight: 119.12 g/mol
tryptophan → triptofan
Tryptophan is hydrophobic amino acids with aromatic side chain. Tryptophan is large aromatic residue that is normally found buried in the interior of a protein and is important for protein stability. Tryptophan has the largest side chain and is the least common amino acid in proteins. It has spectral properties that make it the best inherent probe for following protein folding and conformational changes associated with biochemical processes. It is an essential amino acid, which means that humans cannot synthesize it, so it must be ingested.
- Abbreviations: Trp, W
- IUPAC name: 2-amino-3-(1H-indol-3-yl)propanoic acid
- Molecular formula: C11H12N2O2
- Molecular weight: 204.23 g/mol
tyrosine → tirozin
Tyrosine is hydrophobic amino acids with aromatic side chain. Tyrosine is large aromatic residue that is normally found buried in the interior of a protein and is important for protein stability. Tyrosine has special properties since its hydroxyl side chain may function as a powerful nucleophile in an enzyme active site (when ionized) and is a common site for phosphorylation in cell signaling cascades. Tyrosine absorbs ultraviolet radiation and contributes to the absorbance spectra of proteins. It is not essential (or semi-essential) to the human diet, since it is synthesized in the body from other metabolites.
- Abbreviations: Tyr, Y
- IUPAC name: 2-amino-3-(4-hydroxyphenyl)propanoic acid
- Molecular formula: C9H11NO3
- Molecular weight: 181.19 g/mol
valine → valin
Valine is hydrophobic amino acids with aliphatic side chain. It is a member of the branched-chain amino acid family, along with leucine and isoleucine. Valine differs from threonine by replacement of the hydroxyl group with a methyl substituent, but they are of roughly the same shape and volume. The nonpolar hydrophobic amino acids tend to cluster together within proteins, stabilizing protein structure by means of hydrophobic interactions. Valine is an essential amino acid, which means that it cannot be synthesized in the body and must be obtained through dietary sources.
- Abbreviations: Val, V
- IUPAC name: 2-amino-3-methylbutanoic acid
- Molecular formula: C5H11NO2
- Molecular weight: 117.15 g/mol
Citing this page:
Generalic, Eni. "Strukturna formula." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table