electrical double layer → električni dvosloj
Electrical double layer is the structure of charge accumulation and charge separation that always occurs at the interface when an electrode is immersed into an electrolyte solution. The excess charge on the electrode surface is compensated by an accumulation of excess ions of the opposite charge in the solution. The amount of charge is a function of the electrode potential. This structure behaves essentially as a capacitor. There are several theoretical models that describe the structure of the double layer. The three most commonly used ones are the Helmholtz model, the Gouy-Chapman model, and the Gouy-Chapman-Stern model.
electrochemical cell → elektrokemijski članak
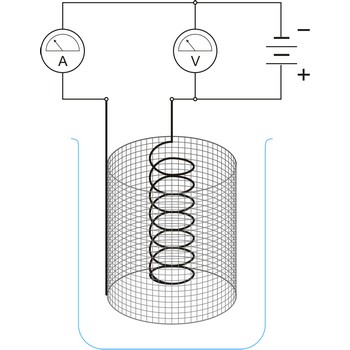
Electrochemical cell is a device that converts chemical energy into electrical energy or vice versa when a chemical reaction is occurring in the cell. It consist of two electronically conducting phases (e.g., solid or liquid metals, semiconductors, etc) connected by an ionically conducting phase (e.g. aqueous or non-aqueous solution, molten salt, ionically conducting solid). As an electric current passes, it must change from electronic current to ionic current and back to electronic current. These changes of conduction mode are always accompanied by oxidation/reduction reactions.
An essential feature of the electrochemical cell is that the simultaneously occurring oxidation-reduction reactions are spatially separated. E.g., in a spontaneous chemical reaction during the oxidation of hydrogen by oxygen to water, electrons are passed directly from the hydrogen to the oxygen.
In contrast, in the spontaneous electrochemical reaction in a galvanic cell the hydrogen is oxidised at the anode by transferring electrons to the anode and the oxygen is reduced at the cathode by accepting electrons from the cathode. The ions produced in the electrode reactions, in this case positive hydrogen ions and the negative hydroxyl (OH-) ions, will recombine in the solution to form the final product of the reaction: water. During this process the electrons are conducted from the anode to the cathode through an outside electric circuit where the electric current can drive a motor, light a light bulb, etc. The reaction can also be reversed: water can be decomposed into hydrogen and oxygen by the application of electrical power in an electrolytic cell.
electrochemical series → elektrokemijski niz
Electrochemical series is a series of chemical elements arranged in order of their standard electrode potentials. The hydrogen electrode
is taken as having zero electrode potential. An electrode potential is, by definition, a reduction potential.
Elements that have a greater tendency than hydrogen to lose electrons to their solution are taken as electropositive; those that gain electrons from their solution are below hydrogen in the series and are called electronegative.
The series shows the order in which metals replace one another from their salts; electropositive metals will replace hydrogen from acids.
electrogravimetry → electrogravimetrija
Electrogravimetry is an electroanalytical technique in which the substance to be determined (usually a metal) is deposited out on an electrode which is weighed before and after the experiment. The potential of the electrode must be carefully chosen to ensure that only the metal do be determined will deposit.
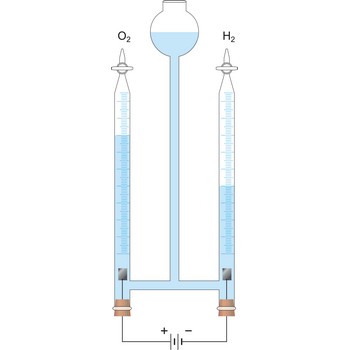
electrolysis → elektroliza
Electrolysis is the decomposition of a substance as a result of passing an electric current between two electrodes immersed in the sample.
electrophoresis → elektroforeza
Electrophoresis is a technique for the analysis and separation of colloids, based on the movement of charged colloidal particles in an electric field. The migration is toward electrodes of charge opposite to that of the particles. The rate of migration of the particles depends on the field, the charge on the particles, and on other factors, such as the size and shape of the particles.
Electrophoresis is important in the study of proteins. The acidity of the solution can be used to control the direction in which a protein moves upon electrophoresis.
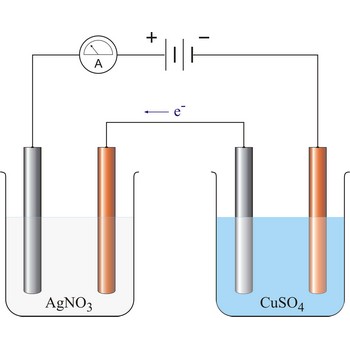
electroplating → galvaniziranje
Electroplating (also called electrodeposition) is the deposition of a metallic coating onto an object by putting a negative charge onto the object and immersing it into a solution which contains a salt of the metal to be deposited. The metallic ions of the salt carry a positive charge and are attracted to the part. When they reach it, the negatively charged part provides the electrons to reduce the positively charged ions to metallic form.
Typically, a brass or nickel object is coated with a layer of silver by making use of electrolysis of a silver solution, using the object to be coated as the cathode. The anode consist of pure silver, and the cathode is the object to be plated. The electrolyte is a mixure of silver nitrate with potassium cyanide. The reactions are:
The cyanide ensures a low concentration of silver ions, a condition for providing the best plating results.
Erlenmeyer flask → Erlenmeyerova tikvica
Erlenmeyer flask is a glass container which has a narrow, cylindrical mouth and a cone-shaped main body that ends in a wide, flat bottom. It was named after its inventor, a German chemist Richard Erlenmeyer (1825-1909). Erlenmeyer flasks are most often used in titrations to hold the liquid that is being titrated.
Faraday’s laws of electrolysis → Faradayevi zakoni elektrolize
Faraday’s laws of electrolysis are two laws found by British chemist and physicist Michael Faraday (1791-1867) in his experiments on electrolysis:
1. The quantity of matter extracted on the electrode is proportional to the quantity of charge (Q = I·t) which has flown in electrolysis time.
where z = number of electrons changed in reaction and F = Faraday’s constant which equals 96 487 C mol-1.
2. The masses of the elements liberated by the same quantity of electricity are directly proportional to their chemical equivalents.
96 487 C will discharge 1 mol Ag and 1/2 mol Cu. The relevant half reactions are:
Citing this page:
Generalic, Eni. "Staklena elektroda." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table