glycogen → glikogen
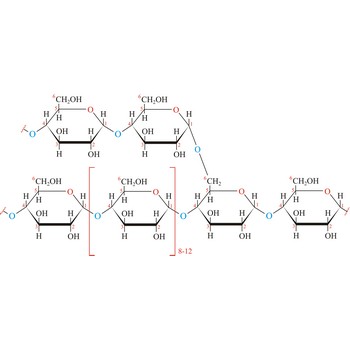
Glycogen (animal starch) is a polysaccharide that serves the same energy storage function in animals that starch serves in plants. Dietary carbohydrates not needed for immediate energy are converted by the body to glycogen for long term storage (principally in muscle and liver cells). Like amylopectin found in starch, glycogen is a polymer of α(1→4)-linked subunits of glucose, with α(1→6)-linked branches. Glycogen molecules are larger than those of amylopectin (up to 100 000 glucose units) and contain even more branches. Branch points occur about every 10 residues in glycogen and about every 25 residues in amylopectin. The branching also creates lots of ends for enzyme attack and provides for rapid release of glucose when it is needed.
mineral oils → mineralna ulja
Mineral oils are oily liquids that are composed of hydrocarbons and are obtained as a product of petroleum, tar, coal, wood etc. distillation. They are used as lubricants.
mineralization → mineralizacija
Mineralization is a process in which organic compounds (e.g. dead plant or animal material) are converted to inorganic compounds (e.g. nitrate, carbon dioxide).
mineralogy → mineralogija
Mineralogy is a branch of geology that studies minerals: their structure and properties and the ways of distinguishing them.
miscible matter → mješljiva tvar
Miscible matter is capable of being mixed in any ratio with another matter without separation of two phases.
glycoside → glikozid
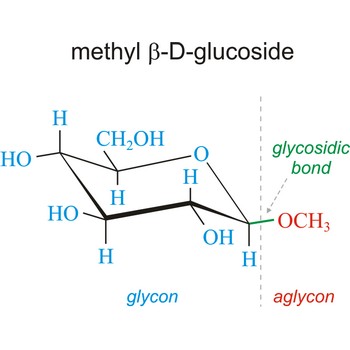
Glycoside is one of a group of organic compounds in which a sugar group is bonded through its anomeric carbon to another group via a glycosidic bond. The sugar group is known as the glycon and the non-sugar group as the aglycon. According to the IUPAC definition, all disaccharides and polysaccharides are glycosides where the aglycone is another sugar.
In the free hemiacetal form, sugars will spontaneously equilibrate between the α and β anomers. However, once the glycosidic bond is formed, the anomeric configuration of the ring is locked as either α or β. Therefore, the alpha and beta glycosides are chemically distinct. They will have different chemical, physical, and biological properties. Many glycosides occur abundantly in plants, especially as flower and fruit pigments.
The term glycoside was later extended to cover not only compounds in which the anomeric hydroxy group is replaced by a group -OR, but also those in which the replacing group is -SR (thioglycosides), -SeR (selenoglycosides), -NR1R2 (N-glycosides), or even -CR1R2R3 (C-glycosides). Thioglycoside and selenoglycoside are legitimate generic terms; however the use of N-glycoside, although widespread in biochemical literature, is improper and not recommended here (glycosylamine is a perfectly acceptable term). C-Glycoside is even less acceptable. All other glycosides are hydrolysable; the C-C bond of C-glycosides is usually not. The use and propagation of names based on C-glycoside terminology is therefore strongly discouraged.
glycosidic bond → glikozidna veza
Glycosidic bond ia a bond between the glycosyl group, the structure obtained by removing the hydroxy group from the hemiacetal function of a monosaccharide, and the -OR group (which itself may be derived from a saccharide and chalcogen replacements thereof (RS–, RSe–). The terms N-glycosides and C-glycosides are misnomers and should not be used. The glycosidic bond can be α or β in orientation, depending on whether the anomeric hydroxyl group was α or β before the glycosidic bond was formed and on the specificity of the enzymatic reaction catalyzing their formation. Once the glycosidic bond is formed, the anomeric configuration of the ring is locked as either α or β. Specific glycosidic bonds therefore may be designated α(1→4), β(1→4), α(1→6), and so on. Cellulose is formed of glucose molecules linked by β(1→4)-glycosidic bonds, whereas starch is composed of α(1→4)-glycosidic bonds.
molar enthalpy of evaporation → molarna entalpija isparavanja
Molar enthalpy of evaporation (Δl gH) is a change of enthalpy during evaporation divided by molarity of a liquid, and is equal to the heat energy spent when the evaporation is conducted under constant pressure, Δl gH=Q.
Citing this page:
Generalic, Eni. "S.t.p.." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table