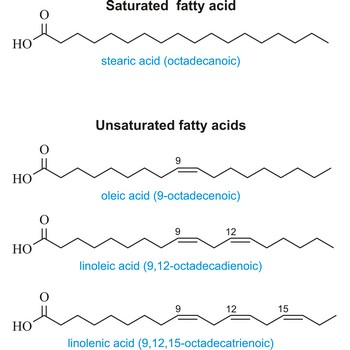
fatty acid → masna kiselina
Fatty acids are aliphatic monocarboxylic acids characterized by a terminal carboxyl group (R-COOH). The higher members of this series of acids occur in nature in the combined form of esters of glycerol (fats), and hence all acids of this family are called fatty acids. Natural fatty acids commonly have a chain of 4 to 28 carbons (usually unbranched and even-numbered), which may be saturated or unsaturated. The most important of saturated fatty acids are butyric (C4), lauric (C12), palmitic (C16), and stearic (C18). The most common unsaturated acids are oleic, linoleic, and linolenic (all C18).
The physical properties of fatty acids are determined by the chain length, degree of unsaturation, and chain branching. Short-chain acids are pungent liquids, soluble in water. As the chain length increases, melting points are raised and water-solubility decreases. Unsaturation and chain branching tend to lower melting points.
Fehling’s test → Fehlingova otopina
Fehling’s test is a chemical test to detect reducing sugars and aldehydes in a solution, devised by the German chemist Hermann Christian von Fehling (1812-1885). Fehling’s solution consists of Fehling’s A (copper(II) sulphate solution) and Fehling’s B (sodium tartarate solution), equal amounts of which are added to the test solution. After boiling, a positive result is indicated by the formation of a brick-red precipitate of copper(I) oxide. Methanal, being a strong reducing agent, also produces copper metal; ketones do not react. The test is now rarely used, having been replaced by Benedict’s test.
ferromagnetism → feromagnetizam
Ferromagnetism is a type of magnetism in which the magnetic moments of atoms in a solid are aligned within domains which can in turn be aligned with each other by a weak magnetic field. The total magnetic moment of a sample of the substance is the vector sum of the magnetic moments of the component domains. In an unmagnetized piece of ferromagnetic material the magnetic moments of the domains themselves are not aligned; when an external field is applied those domains that are aligned with the field increase in size at the expense of the others. Ferromagnetic materials can retain their magnetisation when the external field is removed, as long as the temperature is below a critical value, the Curie temperature. They are characterised by a large positive magnetic susceptibility.
latent heat → latentna toplina
Latent heat (L) is the quantity of heat absorbed or released when a substance changes its physical phase at constant temperature (e.g. from solid to liquid at the melting point or from liquid to gas at the boiling point).
lattice → kristalna rešetka
Crystal lattice is a three-dimensional array of points that embodies the pattern of repetition in a crystalline solid. Don’t mix up atoms with lattice points: lattice points are infinitesimal points in space - atoms are physical objects.
lattice constants → konstante kristalne rešetke
Lattice constants are parameters specifying the dimensions of a unit cell in a crystal lattice, specifically the lengths of the cell edges and the angles between them.
lattice energy → energija kristalne rešetke
Lattice energy is the energy per ion pair required to separate completely the ions in a crystal lattice at a temperature of absolute zero.
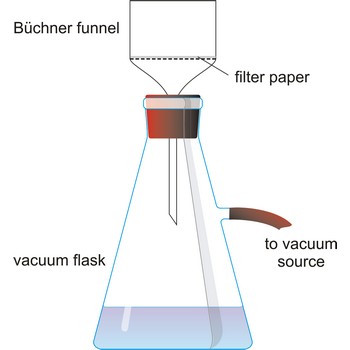
filter flask → boca za odsisavanje
Filter flask, also known as a vacuum flask, is a flask with a side arm to which a vacuum can be applied. It usually have heavy side walls to withstand high vacuum.
filtration → filtriranje
Filtration is a procedure in which liquids are separated from the precipitate by passing a suspension through the filter. The precipitate remains on the filter and through it the filtrate passes. Gaseous heterogeneous mixtures can also be filtrated.
law of chemical equilibrium → zakon o kemijskoj ravnoteži
Law of chemical equilibrium (also called the law of mass action) states that the rate at which a substance reacts is proportional to its active mass (i.e. to its molar concentration). Thus, the velocity of a chemical reaction is proportional to the product of the concentration of the reactants.
Citing this page:
Generalic, Eni. "S.t.p.." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table