conjugated acid → konjugirana kiselina
Conjugated acid is a particle that develops after a base receives a proton.
glacial acetic acid → ledena octena kiselina
Glacial acetic acid (CH3COOH) is the pure compound, as distinguished from the usual water solutions known as acetic acid. It is a colorless liquid or crystalline substance (melting point 16.6 °C) with a pungent, vinegar odor.
deoxyribonucleic acid → dezoksiribonukleinska kiselina
Deoxyribonucleic acid (DNA) is a nucleic acid with 2-deoxy-D-ribose as the sugar in its nucleotides. DNA contains encoded genetic information, specifically templates for the synthesis of all of an organism’s proteins and enzymes.
DNA was first identified in the 1869 by Swiss chemist Friedrich Miescher (1844-1895). In 1953, American biologist James Dewey Watson (1928-) and English physicist Francis Harry Compton Crick (1916–2004) had discovered that DNA occurs in the cell as a double helix, with two long strands of the molecule wound around each other, and further that the chemical structure of the molecule dictates that adenine (A) always aligns or pairs with thymine (T), and cytosine (C) always pairs with guanine (G). It is this base pairing that allows DNA in a cell to copy itself, and transfer its information to a new cell. The diameter of the helix is 2.0 nm and there is a residue on each chain every 0.34 nm in the z direction. The angle between each residue on the same strand is 36°, so that the structure repeats after 10 residues (3.4 nm) on each strand.
Lewis acid → Lewisova kiselina
Lewis acid is an agent capable of accepting a pair of electrons to form a coordinate bond.
mineral acid → mineralna kiselina
Mineral acid is an acid made from minerals by chemical reaction, e.g. hydrochloric acid is produced from sodium chloride and sulphuric acid is made from sulphur.
monobasic acid → monobazična kiselina
Monobasic acids are acids that have only one replacable hydrogen atom per molecule (HCl, HNO3).
muriatic acid → murijatična kiselina
Muriatic acid is an obsolete name for hydrochloric acid (HCl). Lavoisier coined the name from the Latin word muria meaning brine.
organic acid → organska kiselina
Organic acid is an organic compound which is sour, most often these are carboxylic acids (RCOOH).
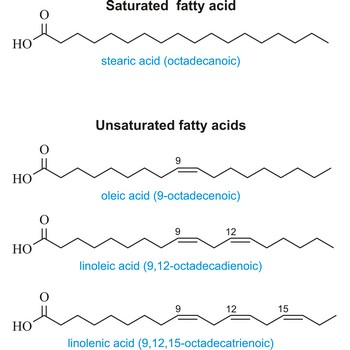
fatty acid → masna kiselina
Fatty acids are aliphatic monocarboxylic acids characterized by a terminal carboxyl group (R-COOH). The higher members of this series of acids occur in nature in the combined form of esters of glycerol (fats), and hence all acids of this family are called fatty acids. Natural fatty acids commonly have a chain of 4 to 28 carbons (usually unbranched and even-numbered), which may be saturated or unsaturated. The most important of saturated fatty acids are butyric (C4), lauric (C12), palmitic (C16), and stearic (C18). The most common unsaturated acids are oleic, linoleic, and linolenic (all C18).
The physical properties of fatty acids are determined by the chain length, degree of unsaturation, and chain branching. Short-chain acids are pungent liquids, soluble in water. As the chain length increases, melting points are raised and water-solubility decreases. Unsaturation and chain branching tend to lower melting points.
weak acid → slaba kiselina
Weak acid is an acid that incompletely dissociated in aqueous solution. Acetic acid is an example of a weak acid
Citing this page:
Generalic, Eni. "Ribonucleic acids." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. 2 Apr. 2025. <https://glossary.periodni.com>.
Glossary
Periodic Table