leucine → leucin
Leucine is hydrophobic amino acids with aliphatic side chain. It has one additional methylene group in its side chain compared with valine. The nonpolar hydrophobic amino acids tend to cluster together within proteins, stabilizing protein structure by means of hydrophobic interactions. Leucine is an essential amino acid, which means that humans cannot synthesize it, so it must be ingested.
- Abbreviations: Leu, L
- IUPAC name: 2-amino-4-methylpentanoic acid
- Molecular formula: C6H13NO2
- Molecular weight: 131.17 g/mol
phenylalanine → fenilalanin
Phenylalanine is hydrophobic amino acids with aromatic side chain. It is quite hydrophobic and even the free amino acid is not very soluble in water. Phenylalanine is large aromatic residue that is normally found buried in the interior of a protein and is important for protein stability. It is an essential amino acid, which means that humans cannot synthesize it, so it must be ingested.
- Abbreviations: Phe, F
- IUPAC name: 2-amino-3-phenylpropanoic acid
- Molecular formula: C9H11NO2
- Molecular weight: 165.19 g/mol
monosaccharide → monosaharid
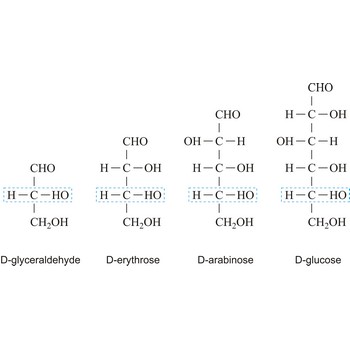
Monosaccharides are carbohydrates, with the general formula Cn(H2O)n, that cannot be decomposed to a simpler carbohydrates by hydrolysis.
Depending on whether the molecule contains an aldehyde group (-CHO) or a ketone group (-CO-) monosaccharide can be a polyhydroxy aldehyde (aldose) or a polyhydroxy ketone (ketose). These aldehyde and ketone groups confer reduction properties on monosaccharides. They are also classified according to the number of carbon atoms they contain: trioses have three carbon atoms, tetroses four, pentoses five, hexoses six, heptoses seven, etc. These two systems of classification are often combined. For example, a six-carbon polyhydroxy aldehyde such as D-glucose is an aldohexose, whereas a six-carbon polyhydroxy ketone such as D-fructose is a ketohexose.
The notations D and L are used to describe the configurations of carbohydrates. In Fischer projections of monosaccharides, the carbonyl group is always placed on top (in the case of aldoses) or as close to the top as possible (in the case of ketoses). If the OH group attached to the bottom-most asymmetric carbon (the carbon that is second from the bottom) is on the right, then the compound is a D-sugar. If the OH group is on the left, then the compound is an L-sugar. Almost all sugars found in nature are D-sugars.
Monosaccharides can exist as either straight-chain or ring-shaped molecules. During the conversion from straight-chain form to cyclic form, the carbon atom containing the carbonyl oxygen, called the anomeric carbon, becomes a chiral center with two possible configurations (anomers), α and β. When the stereochemistry of the first carbon matches the stereochemistry of the last stereogenic center the sugar is the α-anomer when they are opposite the sugar is the β-anomer.
polystyrene → polistiren
Polystyrene is a vinyl polymer. Structurally, it is a long hydrocarbon chain, with a phenyl group attached to every other carbon atom. Polystyrene is produced by free radical vinyl polymerization, from the monomer styrene. Polystyrene or Styrofoam is used in the construction industry as insulating material and for production of containers.
saturated fatty acid → zasićena masna kiselina
Saturated fatty acid is a fatty acid carrying the maximum possible number of hydrogen atoms (It doesn’t have any double bounds in the alkyl chain). The most important of these are:
| Butyric (butanoic acid) | CH3(CH2)2COOH |
| Lauric (dodecanoic acid) | CH3(CH2)10COOH |
| Myristic (tetradecanoic acid) | CH3(CH2)12COOH |
| Palmitic (hexadecanoic acid) | CH3(CH2)14COOH |
| Stearic (octadecanoic acid) | CH3(CH2)16COOH |
| Arachidic (eicosanoic acid) | CH3(CH2)18COOH |
thermosetting plastic → termostabilna plastika
Thermosetting plastics (thermosets) refer to a range of polymer materials that cure, through the addition of energy, to a stronger form. The energy may be in the form of heat (rubber), through a chemical reaction (two part epoxy), or irradiation. Thermoset materials are usually liquid or malleable prior to curing, and designed to be molded into their final form, or used as adhesive.
Thermoset polymer resins transformed into plastics or rubbers by cross-linking into a rigid, 3-D structure. A thermoset material cannot be melted and re-molded after it is cured.
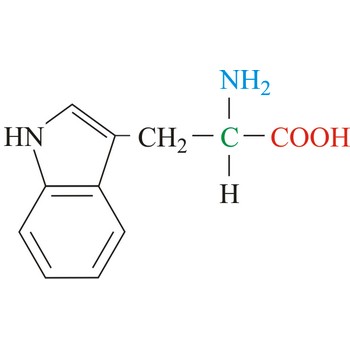
tryptophan → triptofan
Tryptophan is hydrophobic amino acids with aromatic side chain. Tryptophan is large aromatic residue that is normally found buried in the interior of a protein and is important for protein stability. Tryptophan has the largest side chain and is the least common amino acid in proteins. It has spectral properties that make it the best inherent probe for following protein folding and conformational changes associated with biochemical processes. It is an essential amino acid, which means that humans cannot synthesize it, so it must be ingested.
- Abbreviations: Trp, W
- IUPAC name: 2-amino-3-(1H-indol-3-yl)propanoic acid
- Molecular formula: C11H12N2O2
- Molecular weight: 204.23 g/mol
starch → škrob
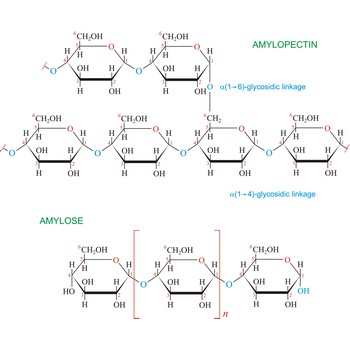
Starch (C6H10O5)x is a polysaccharide used by plants to stockpile glucose molecules. It is the major component of flour, potatoes, rice, beans, corn, and peas. Starch is a mixture of two different polysaccharides: amylose (about 20 %), which is insoluble in cold water, and amylopectin (about 80 %), which is soluble in cold water. Amylose is composed of unbranched chains of D-glucose units joined by α(1→4)-glycosidic linkages. Unlike amylose, which are linear polymers, amylopectin contains α(1→6)-glycoside branches approximately every 25 glucose units.
Starch digestion begins in the mouth via the action of amylase, a digestive enzyme present in saliva. The process is completed in the small intestine by the pancreatic amylase. The final products of starch digestion, glucose molecules, are absorbed into the intestinal bloodstream and transported to the liver. Like most enzymes, glycosidases are highly selective in their action. They hydrolyze only the α-glycoside links in starch and leave the β-glycoside links in cellulose untouched. Starch is important food stuff and is used in adhesives, and sizes, in laundering, pharmacy and medicine.
tyrosine → tirozin
Tyrosine is hydrophobic amino acids with aromatic side chain. Tyrosine is large aromatic residue that is normally found buried in the interior of a protein and is important for protein stability. Tyrosine has special properties since its hydroxyl side chain may function as a powerful nucleophile in an enzyme active site (when ionized) and is a common site for phosphorylation in cell signaling cascades. Tyrosine absorbs ultraviolet radiation and contributes to the absorbance spectra of proteins. It is not essential (or semi-essential) to the human diet, since it is synthesized in the body from other metabolites.
- Abbreviations: Tyr, Y
- IUPAC name: 2-amino-3-(4-hydroxyphenyl)propanoic acid
- Molecular formula: C9H11NO3
- Molecular weight: 181.19 g/mol
unsaturated fatty acid → nezasićena masna kiselina
Unsaturated fatty acid is a fatty acid whose carbon chain can absorb additional hydrogen atoms. Their carbon chain has one or more double or triple valence bond per molecule. The most important of these are:
| Oleic (9-octadecenoic acid) | CH3(CH2)7CH=CH(CH2)7COOH |
| Linoleic (9,12-octadecadienoic acid) | CH3(CHCH2)3(CH2CH=CH)2(CHCH2)7COOH |
| Linolenic (9,12,15-octadecatrienoic acid) | CH3(CH2CH=CH)3(CHCH2)7COOH |
Citing this page:
Generalic, Eni. "Postranični lanac." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table