contaminant → kontaminat
Contaminants are any physical, chemical, biological, or radiological substance or matter in water that may be harmful to human health or which degrade the palatability of water.
bromine → brom
Bromine was discovered by Antoine J. Balard (France) in 1826. The origin of the name comes from the Greek word bromos meaning stench. It is reddish-brown liquid with suffocating, irritating fumes. Gives off poisonous vapour. Causes severe burns. Oxidizer. Bromine occurs in compounds in sea water. It was once used in large quantities to make a compound that removed lead compound build up in engines burning leaded gasoline. Now it is primarily used in dyes, disinfectants and photographic chemicals.
bronze → bronca
Bronze is an alloy made primarily of copper and tin. It may contain as much as 25 % tin. Bronzes with 10 % or more tin are harder, stronger, and resistant to corrosion. As bronze weathers, a brown or green film forms on the surface. This film inhibits corrosion. Silicon or aluminium is often added to bronze to improve resistance to corrosion. Phosphorus, lead, zinc, and other metals may be added for special purposes. The alloy is hard and easily cast and is extensively used in bearings, valves and other machine parts.
Bronze was one of the first alloys developed by ancient metal workers. The Bronze Age occurred in Europe around 2200 to 700 BC. Bronze was used for weapons such as spearheads, swords, and knives. Since ancient times, bronze has been the most popular metal for casting statues and other art objects.

The term bronze has been adopted commercially for many copper-rich alloys that contain little or no tin but are similar in colour to bronze, including aluminium bronze, manganese bronze, and silicon bronze. Aluminium bronze is used to make tools and, because it will not spark when struck. Manganese bronze is actually a brass that contains manganese. It is often used to make ship propellers because it is strong and resists corrosion by sea water.
decomposing → raščinjavanje
Decomposing in analytical chemistry means that a certain substance is converted, by melting it with a suitable melting medium (sodium carbonate, sodium hydroxide, sodium peroxide, ...) in the kind of compound which will afterwards that dissolve in water, acid or base very easily.
detergent → deterdžent
Detergent is a substance added to water to improve its cleaning properties. Although water is a powerful solvent for many compounds, it will not dissolve grease and natural oils. Detergents are compounds that cause such nonpolar substances to go into solution in water. Soap is the original example, owing its action to the presence of ions formed from long-chain fatty acids ion (e.g. stearat ion, CH3(CH2)16COO-).
caesium → cezij
Caesium was discovered by Robert Bunsen and Gustav Kirchhoff (Germany) in 1860. The origin of the name comes from the Latin word caesius meaning sky blue or heavenly blue. It is very soft, light grey, ductile metal. Reacts readily with oxygen. Reacts explosively with water. Caesium is found in pollucite [(Cs4Al4Si9O26)·H2O] and as trace in lepidolite. Used as a ’getter’ to remove air traces in vacuum and cathode-ray tubes. Also used in producing photoelectric devices and atomic clocks. Since it ionises readily, it is used as an ion rocket motor propellant.
calcium → kalcij
Calcium was discovered by Sir Humphry Davy (England) in 1808. The origin of the name comes from the Latin word calix meaning lime. It is fairly hard, silvery-white metal. Exposed surfaces form oxides and nitrides. Reacts with water and oxygen. Occurs only in compounds. Calcium is obtained from minerals like chalk, limestone and marble. Pure metal is produced by replacing the calcium in lime (CaCO3) with aluminium in hot, low pressure retorts. Used by many forms of life to make shells and bones. Virtually no use for the pure metal, however two of its compounds are, lime (CaO) and gypsum (CaSO4), are in great demand by a number of industries.
carbohydrate → ugljikohidrat
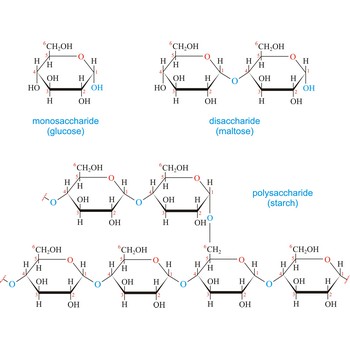
Carbohydrates (often called carbs for short) are polyhydroxy aldehydes or ketones, or substances that yield such compounds on hydrolysis. They are also known as saccharides, a term derived from the Latin word saccharum for sugar. Carbohydrates are the most abundant class of compounds in the biological world, making up more than 50 % of the dry weight of the Earth’s biomass. Every type of food we eat can have its energy traced back to a plant. Plants use carbon dioxide and water to make glucose, a simple sugar, in photosynthesis. Other carbohydrates such as cellulose and starch are made from the glucose. Light from the sun is absorbed by chlorophyll and this is converted to the energy necessary to biosynthesize carbohydrates
The term carbohydrate was applied originally to monosaccharides, in recognition of the fact that their empirical composition can be expressed as Cx(H2O)y. Later structural studies revealed that these compounds were not hydrates but the term carbohydrate persists.
Carbohydrates are generally classed as either simple or complex. Simple sugars, or monosaccharides, are carbohydrates that can’t be converted into smaller subunits by hydrolysis. Complex carbohydrates are made of two (disaccharides) or more (oligosaccharides, polysaccharides) simple sugars linked together by acetal (glycosidic) bonds and can be split into the former by hydrolysis.
dilution → razrjeđivanje
Dilution is the action of diluting or reducing the strength or concentration of a liquid, usually by the addition of water.
Citing this page:
Generalic, Eni. "Permanent hardness in water." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table