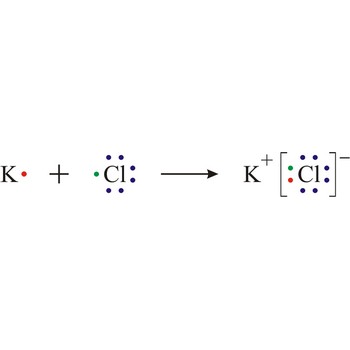laminar flow → laminarno strujanje
Laminar flow is a smooth, uniform, non-turbulent flow of a gas or liquid in parallel layers, with little mixing between layers. It is characterised by small values of the Reynolds number.
lanthanides → lantanoidi
Lanthanides (lanthanons, lanthanoids or rare-earth elements) are a series of fourteen elements in the periodic table, generally considered to range in proton number from cerium to lutetium inclusive. It was convenient to divide these elements into the cerium group or light earth: cerium (Ce), praseodymium (Pr), neodymium (Nd), promethium (Pm), samarium (Sm), europium (Eu); and the yttrium group or heavy earths: gadolinium (Gd), terbium (Tb), dysprosium (Dy), holmium (Ho), erbium (Er), thulium (Tm), ytterbium (Yb) i lutetium (Lu). The position of lanthanum is somewhat equivocal and, although not itself a lanthanide, it is often included with them for comparative purpose. The lanthanides are sometimes simply called the rare earths. Apart from unstable Pm, the lanthanides are actually not rare. Cerium is the 26. most abundant of all elements, 5 times as abundant as Pb. All are silvery very reactive metals.
lateral chain → postranični lanac
Lateral chain is a shorter chain of hydrocarbons which is connected to the main chain of hydrocarbon.
leucine → leucin
Leucine is hydrophobic amino acids with aliphatic side chain. It has one additional methylene group in its side chain compared with valine. The nonpolar hydrophobic amino acids tend to cluster together within proteins, stabilizing protein structure by means of hydrophobic interactions. Leucine is an essential amino acid, which means that humans cannot synthesize it, so it must be ingested.
- Abbreviations: Leu, L
- IUPAC name: 2-amino-4-methylpentanoic acid
- Molecular formula: C6H13NO2
- Molecular weight: 131.17 g/mol
Lewis structure → Lewisova struktura
Lewis structure is the representation of the electron arrangement in atoms, ions, or molecules by showing the valence electrons as dots placed around the symbols for the elements.
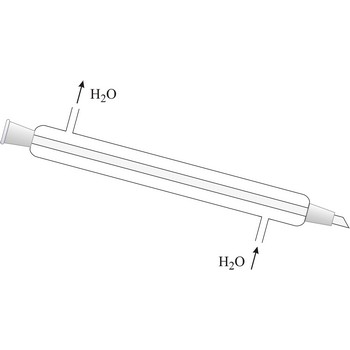
Liebig condenser → Liebigovo hladilo
Liebig condenser is used for condensing of vapours that pass trough the centre tube. It is cooled with water that passes in the outer tube (shell around the centre tube) in the opposite direction than the one of hot vapour. Though named after the German chemist Justus von Liebig (1803-1873), he cannot be given credit for having invented it because it had already been in use for some time before him.
ligand → ligand
Ligand is an ion (F-, Cl-, Br-, I-, S2-, CN-, NCS-, OH-, NH2-) or molecule (NH3, H2O, NO, CO) that donates a pair of electrons to a metal atom or ion in forming a coordination complex. The main way of classifying ligands is by the number of points at which they are attached to, or bound to, the metal center. This is the denticity. Ligands with one potential donor atom are monodentate. Polydentate ligand is a ligand that is attached to a central metal ion by bonds from two or more donor atoms. Ligands with more than one potential donor atom are known as ambidentate, such as the thiocyanate ion, NCS-, which can bind to the metal center with either the nitrogen or sulphur atoms. Chelating ligands are those polydentate ligands which can form a ring including the metal atom.
linear molecular geometry → linearna geometrija molekule
Linear molecule is a molecule in which atoms are deployed in a straight line (under 180° angle). Molecules with an linear electron pair geometries have sp hybridization at the central atom. An example of linear electron pair and molecular geometry are carbon dioxide (O=C=O) and beryllium hydride BeH2.
Citing this page:
Generalic, Eni. "Gallery/images.php." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table