rotational inertia → moment tromosti
Rotational inertia of a body is defined as
for a system of discrete particles (each of mass mi), and as
for a body with continuously distributed mass (dm is the mass element). ri and r represent the perpendicular distance from the axis of rotation to the mass element of the body.
SI unit for rotational inertia is kg m2.
seawater → more
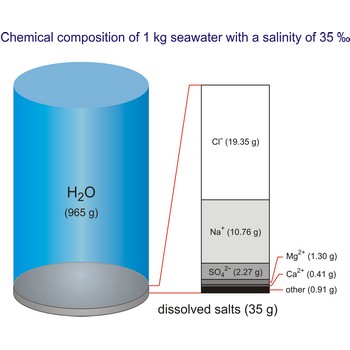
Seawater is a complex mixture of 96.5 % water, 3.5 % salts, and smaller amounts of other substances, including dissolved inorganic and organic materials, particulates, and a few atmospheric gases. The world's oceans cover nearly 71 % (361 840 000 km2) of the Earth's surface (510 100 000 km2), with an average depth of 3 682.2 m.
The density of seawater is higher than that of fresh water because of its higher salinity. Seawater's freezing point is lower than that of pure water and its boiling point is higher. The average salinity of the ocean is 35 ‰, which means that for every kilograms of water, there are 35 g of salt. The relative abundance of the major salts in seawater are constant regardless of the ocean. Only six elements and compounds comprise about 99 % of sea salts: chlorine (Cl-), sodium (Na+), sulfur (SO42-), magnesium (Mg2+), calcium (Ca2+), and potassium (K+).
specific weight → specifična težina
Specific weight (γ) is defined as the ratio between the weight of a mass element, Δm, and the volume, ΔV, occupied by that element. As density (average) is defined as the ratio of a mass element and its volume, specific weight is equal to:
where g is gravitational acceleration.
spin → spin
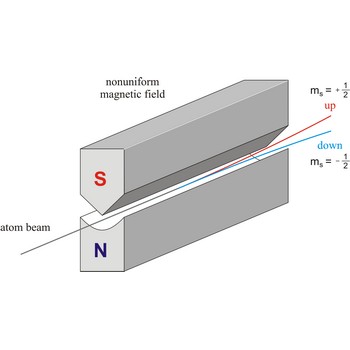
Spin is the intrinsic angular momentum of an elementary particle, or system of particles such as nucleus, that is also responsible for the magnetic moment; or, a particle or nucleus possessing such a spin. The spins of nuclei have characteristic fixed values. Pairs of neutrons and protons align to cancel out their spins, so that nuclei with an odd number of neutrons and/or protons will have a net non-zero rotational component characterized by a non-zero quantum nuclear spin number.
Stern-Gerlach experiment: a beam of silver atoms is split into two beams when it traverses a nonuniform magnetic field. Atoms with spin quantum number ms=+1/2 follow one trajectory, and those with ms=+1/2 follow another.
stoichiometry → stehiometrija
Stoichiometry is the relative proportions elements from compounds or in which substances react. Every chemical reaction has its characteristic proportions. For example, when methane unites with oxygen in complete combustion, 1 mol of methane requires 2 mol of oxygen.
At the same time, 1 mol of carbon dioxide and 2 mol of water are formed as reaction products.
Alternatively, 16 g of methane and 64 g of oxygen produce 44 g of carbon dioxide and 36 g of water.
The stoichiometric relationship between the products and reactants can be used to in calculations.
structural formula → strukturna formula
Structural formula is a two dimensional representations of the arrangement of the atoms in molecules. Atoms are represented by their element symbols and covalent bonds are represented by lines. The symbol for carbon is often not drawn.
technetium → tehnecij
Technetium was discovered by Carlo Perrier and Emilio Segre (Italy) in 1937. The origin of the name comes from the Greek word technikos meaning artificial. It is silvery-grey metal. Resists oxidation but tarnishes in moist air and burns in high oxygen environment. First synthetically produced element. Radioactive. Technetium is made first by bombarding molybdenum with deuterons (heavy hydrogen) in a cyclotron. Added to iron in quantities as low as 55 part-per-million transforms the iron into a corrosion-resistant alloy.
transition metal → prijelazni element
This group of metals is distinguished from other metals not by their physical properties, but by their electronic structure. Transition metals are elements characterized by a partially filled d subshell. The First Transition Series comprises scandium (Sc), titanium (Ti), vanadium (V), chromium (Cr), manganese (Mn), iron (Fe), cobalt (Co), nickel (Ni) and copper (Cu). The Second and Third Transition Series include the lanthanides and actinides, respectively.
The transition metals are noted for their variability in oxidation state. Thus, manganese has two electrons in its outside shell and five electrons in the next shell down, and exhibits oxidation states of +1, +2, +3, +4, +5, +6, and +7.
They are also characterised by the fact that well into the series, going from left to right, the properties of the succeeding metals do not differ greatly from the preceding ones.
ununbium → ununbij
Ununbium was discovered by S. Hofmann et al. collaboration at the Heavy Ion Research Laboratory (Gesellschaft für Schwerionenforschung, GSI) in Darmstadt, Germany in February 1996. The new element has not yet been officially named, but it is known as ununbium, according to the system designated by the IUPAC for naming new elements. It is synthetic radioactive metal. Using the electromagnetic velocity filter SHIP, fusion-like residues of the reaction of 70Zn with enriched 208Pb targets were measured. Two chains of localized alpha-emitters were identified as originating with 277112 + 1n.
ununquadium → ununkvadij
The discovery of ununquadium was reported informally in January 1999 following experiments towards the end of December 1998 involving scientists at Dubna (Joint Institute for Nuclear Research) in Russia and the Lawrence Livermore National Laboratory, USA. The new element has not yet been officially named, but it is known as ununquadium, according to the system designated by the IUPAC for naming new elements. It is synthetic radioactive metal. Only few atoms of element 114 (289114) has ever been made (through a nuclear reaction involving fusing a calcium atom with a plutonium atom) isolation of an observable quantity has never been achieved.
Citing this page:
Generalic, Eni. "Elementi rijetkih zemalja." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table