halogens → halogeni elementi
Halogens are the elements fluorine (F) chlorine (Cl), bromine (Br), iodine (I), and astatine (At). They are non-metals, and make up part of the 17 group in the periodic table. Compounds of these elements are called halogenides or halides.
The halogens all have a strong unpleasant odour and will burn flesh. They do not dissolve well in water. The five elements are strongly electronegative. They are oxidising agents, with fluorine being the strongest and astatine being the weakest. They react with most metals and many non-metals.
Halogens form molecules which consist of atoms covalently bonded. With increasing atomic weight there is a gradation in physical properties. For example: Fluorine is a pale green gas of low density. Chlorine is a greenish-yellow gas 1.892 times as dense as fluorine. Bromine is a deep reddish-brown liquid which is three times as dense as water. Iodine is a grayish-black crystalline solid with a metallic appearance. And astatine is a solid with properties which indicate that it is somewhat metallic in character.
hydrosphere → hidrosfera
Hydrosphere (from the Greek for water sphere) is a discontinuous layer of water on, under, and over the Earth's surface. It includes all liquid and frozen surface waters, groundwater held in soil and rock, and atmospheric water vapour. Water continuously circulates between these reservoirs in what is called the hydrologic cycle, which is driven by energy from the Sun.
| Reservoir | V / 106 km3 | w / % |
|---|---|---|
| oceans | 1 370.0 | 97.25 |
| ice caps and glaciers | 29.0 | 2.05 |
| groundwater | 9.5 | 0.68 |
| lakes, rivers | 0.127 | 0.01 |
| soil moisture | 0.065 | 0.005 |
| atmosphere (as liquid equivalent of water vapour) | 0.013 | 0.001 |
| biosphere | 0.0006 | 0.00004 |
| TOTAL | 1 408.7 | 100 |
kelvin → kelvin
Kelvin (K) is the SI base unit of thermodynamic temperature.
The kelvin, unit of thermodynamic temperature, is the fraction 1/273.16 of the thermodynamic temperature of the triple point of water. The unit was named after the British scientist Sir. W. Thompson, Lord Kelvin (1824-1907).kinetic energy → kinetička energija
Kinetic energy (Ek) is associated with the state of motion of a body. It is a scalar property and defined to be
Kinetic energy is most clearly exhibited in gases, in which molecules have much greater freedom of motion than in liquids and solids.
law of conservation of mass → zakon o očuvanju mase
Law of conservation of mass states that no detectable gain or loss in mass occurs in chemical reactions. The state of a substance may change in a chemical reaction, for example, from a solid to a gas, but its total mass will not change. Note that the energy released (exothermic) or adsorbed (endothermic) in a chemical reaction is a result of energy transfer between atoms and their environment.
sublimation → sublimacija
Sublimation is the conversion of a substance from its solid form directly to its gaseous form without the intervening liquid form. Dry ice (frozen carbon dioxide) sublimates at normal room temperature.
melting point → talište
Melting point is the temperature at which a solid becomes a liquid at normal atmospheric pressure.
A more specific definition of melting point (or freezing point) is the temperature at which the solid and liquid phases of a substance are in equilibrium at a specified pressure (normally taken to be atmospheric unless stated otherwise). A pure substance under standard condition of pressure has a single reproducible melting point. The terms melting point and freezing point are often used interchangeably, depending on whether the substance is being heated or cooled.
metal → metal
Metals are materials in which the highest occupied energy band (conduction band) is only partially filled with electrons.
Their physical properties generally include:
- They are good conductors of heat and electricity. The electrical conductivity of metals generally decreases with temperature.
- They are malleable and ductile in their solid state.
- They show metallic lustre.
- They are opaque.
- They have high density.
- They are solids (except mercury)
- They have a crystal structure in which each atom is surrounded by eight to twelve near neighbours
Their chemical properties generally are:
- They have one to four valence electrons.
- They have low ionisation potentials; they readily lose electrons.
- They are good reducing agents.
- They have hydroxides which are bases or amphoteric.
- They are electropositive.
Metallic characteristics of the elements decrease and non-metallic characteristics increase with the increase of valence electrons. Also metallic characteristics increase with the number of electron shells. Therefore, there is no sharp dividing line between the metals and non-metals.
Of the 114 elements now known, only 17 show primarily non-metallic characteristics, 7 others are metalloids, and 89 may be classed as metals.
phase diagram → fazni dijagram
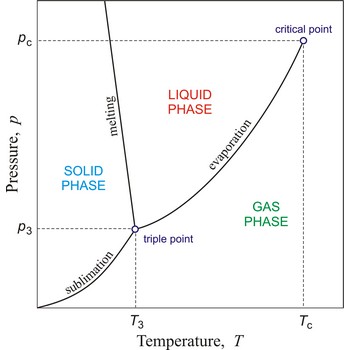
Phase diagram is a graphic representation of the equilibrium relationships between phases (such as vapour-liquid, liquid-solid) of a chemical compound, mixture of compounds, or solution.
The figure shows a typical phase diagram of an element or a simple compound. The stability of solid, liquid and gas phases depends on the temperature and the pressure. The three phases are in equilibrium at the triple point. The gas and liquid phases are separated by a phase transition only below the temperature of the critical point.
saturated fat → zasićena mast
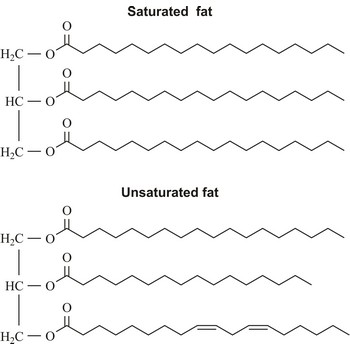
Saturated fats are fats in foods that are solid at room temperature. They come chiefly from animal sources (beef, whole-milk dairy products, dark meat poultry) but also from tropical vegetable oils (coconut, palm).
Citing this page:
Generalic, Eni. "čvrsto agregatno stanje." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table