beta particle → beta-čestica
Beta particle is a charged particle emitted from a radioactive atomic nucleus either natural or manufactured. The energies of beta particles range from 0 MeV to 4 MeV. They carry a single charge; if this is negative, the particle is identical with an electron; if positive, it is a positron.
An unstable atomic nucleus changes into a nucleus of the same mass number but different proton number with the emission of an electron and an antineutrino (or a positron and a neutrino)
blackbody radiation → zračenje crnog tijela
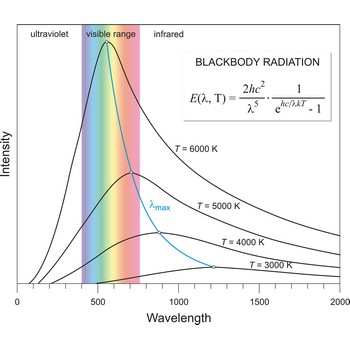
Blackbody radiation is the radiation emitted by a perfect blackbody, i.e., a body which absorbs all radiation incident on it and reflects none. The primary law governing blackbody radiation is the Planck Radiation Law, which governs the intensity of radiation emitted by unit surface area into a fixed direction (solid angle) from the blackbody as a function of wavelength for a fixed temperature. The Planck Law can be expressed through the following equation
where λ is the wavelength, h is Planck’s constant, c is the speed of light, k is the Boltzmann constant, and T is the temperature.
Boltzmann constant → Boltzmannova konstanta
The Boltzmann constant (k or kB) is the physical constant describing the relationship between the thermodynamic temperature and the average kinetic energy of particles in a gas. It equals the molar gas constant R divided by the Avogadro constant NA and has the value 1.380 648 52(79)×10-23 J/K. It is named after the Austrian physicist Ludwig Eduard Boltzmann (1844-1906).
Born-Haber cycle → Born-Haberov kružni proces
Born-Haber cycle is a cycle of reactions used for calculating the lattice energies of ionic crystalline solids. For a compound MX, the lattice energy is the enthalpy of the reaction
The standard enthalpy of formation of the ionic solid is the enthalpy of the reaction
The cycle involves equating this enthalpy (which can be measured) to the sum of the enthalpies of a number of steps proceeding from the elements to the ionic solid. The steps are:
1) Atomization of the metal
2) Atomization of the nonmetal
3) Ionisation of the metal
This is obtained from the ionisation potential.
4) Ionisation of the nonmetal
This is electron affinity.
5) Formation of the ionic solids
Equation of the enthalpies gives
from which ΔHL can be found.
degenerate orbitals → degenerirane orbitale
Degenerate orbitals are orbitals with the same energy. This degeneracy can sometimes be "lifted" by external electric or magnetic fields.
electron spin → elektronski spin
Electron spin (s) is the quantum number, equal to 1/2, that specifies the intrinsic angular momentum of the electron.
electron volt → elektronvolt
Electron volt (eV) is a non-SI unit of energy used in atomic and nuclear physics, equal to approximately 1.602 177×10-19 J. The electron volt is defined as the kinetic energy acquired by an electron upon acceleration through a potential difference of 1 V.
endothermic reaction → endotermna reakcija
Endothermic reactions are the ones in which heat is absorbed and are facilitated by an increase in temperature (ΔH° > 0).
If the reaction is endothermal in one direction, in the opposite direction the reaction is exothermal.
carbohydrate → ugljikohidrat
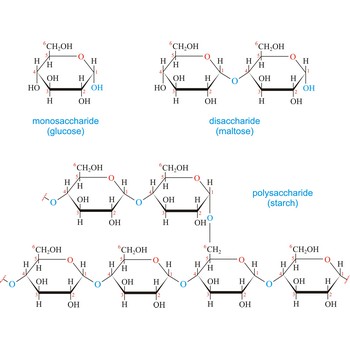
Carbohydrates (often called carbs for short) are polyhydroxy aldehydes or ketones, or substances that yield such compounds on hydrolysis. They are also known as saccharides, a term derived from the Latin word saccharum for sugar. Carbohydrates are the most abundant class of compounds in the biological world, making up more than 50 % of the dry weight of the Earth’s biomass. Every type of food we eat can have its energy traced back to a plant. Plants use carbon dioxide and water to make glucose, a simple sugar, in photosynthesis. Other carbohydrates such as cellulose and starch are made from the glucose. Light from the sun is absorbed by chlorophyll and this is converted to the energy necessary to biosynthesize carbohydrates
The term carbohydrate was applied originally to monosaccharides, in recognition of the fact that their empirical composition can be expressed as Cx(H2O)y. Later structural studies revealed that these compounds were not hydrates but the term carbohydrate persists.
Carbohydrates are generally classed as either simple or complex. Simple sugars, or monosaccharides, are carbohydrates that can’t be converted into smaller subunits by hydrolysis. Complex carbohydrates are made of two (disaccharides) or more (oligosaccharides, polysaccharides) simple sugars linked together by acetal (glycosidic) bonds and can be split into the former by hydrolysis.
catalyst → katalizator
Catalyst is a substance that increases the rate of a chemical reaction without itself undergoing any permanent chemical change. Catalysts that have the same phase as the reactants are homogenous catalysts (e.g. enzymes in biochemical reactions). Those that have a different phase are heterogeneous catalyst (e.g. metals or oxides used in gas reactions).
The catalyst provides an alternative pathway by which the reaction can proceed, in which the activation energy is lower. In thus increases the rate at which the reaction comes to an equilibrium, although it does not alter the position of the equilibrium.
Citing this page:
Generalic, Eni. "Zakon o očuvanju energije." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table