water softener → omekšivač vode
Water softeners are substances which help remove constant water hardness. It reacts with calcium and magnesium salts, creating compounds that do not react with soap.
Knudsen burette → Knudsenova bireta
Knudsen's automatic bulb-burette, developed by the Danish physicist Martin Knudsen (1871-1949), is designed in a way that even routine field analysis in a boat laboratory would provide highly accurate measurements. The burette is filled with a mixture of silver nitrate from reservoir R, located above the burette, by opening the A valve. When the solution crosses the three-way C valve the A valve is closed preventing further solution flow in to the burette. Any extra solution is caught in the W bowl. Turn the C valve, which marks the zero on the scale, in order to allow atmospheric air to enter the burette. Since most open-ocean samples lie in a relatively small chlorinity range, the burette is designed so that much of its capacity is in the bulb (B). This allows the titration to be quick (by quickly releasing contents from the B area) and reduces the error that occurs from the slow drainage along the inner wall of the burette.
Each millimeter is divided in to twenty parts (double millimeter division of the Knudsen burette) which allows for highly accurate measurements (the scale is read up to a precision of 0.005 mL). From 0 to 16 the burette isn't divided, that usually starts from 16 and goes until 20.5 or 21.5. A single double millimeter on a Knudsen burette scale corresponds to one permille of chloride in the seawater sample. This burette can be used for titration of water from all of the oceans and seas, with the exemptions being areas with very low salinity (e.g. the Baltic Sea) and river estuaries which require the use of normal burettes.
piezoelectric effect → piezoelektrični efekt
Piezoelectric effect is voltage produced between surfaces of a solid dielectric (nonconducting substance) when a mechanical stress is applied to it. A small current may be produced as well. The effect, discovered by the French physicist Pierre Curie (1859-1906) in 1883, is exhibited by certain crystals, e.g., quartz and Rochelle salt, and ceramic materials. When a voltage is applied across certain surfaces of a solid that exhibits the piezoelectric effect, the solid undergoes a mechanical distortion. Piezoelectric materials are used in transducers, e.g., phonograph cartridges, microphones, and strain gauges, which produce an electrical output from a mechanical input, and in earphones and ultrasonic radiators, which produce a mechanical output from an electrical input.
potassium → kalij
Potassium was discovered by Sir Humphry Davy (England) in 1807. The origin of the name comes from the Arabic word qali meaning alkali (the origin of the symbol K comes from the Latin word kalium). It is soft, waxy, silver-white metal. Fresh surface has silvery sheen. Quickly forms dull oxide coating on exposure to air. Reacts strongly with water. Reacts with water to give off flammable gas. Reacts violently with oxidants. Occurs only in compounds. Potassium is found in minerals like carnallite [(KMgCl3)·6H2O] and sylvite (KCL). Pure metal is produced by the reaction of hot potassium chloride and sodium vapours in a special retort. Used as potash in making glass and soap. Also as saltpetre, potassium nitrate (KNO3) to make explosives and to colour fireworks in mauve. Vital to function of nerve and muscle tissues.
praseodymium → praseodimij
Praseodymium was discovered by Carl F. Auer von Welsbach (Austria) in 1885. The origin of the name comes from the Greek words prasios didymos meaning green twin. It is silvery white, moderately soft, malleable, ductile metal. Reacts slowly with oxygen. Reacts rapidly with water. Metal ignites and burns readily. Praseodymium is obtained from same salts as neodymium. Used with neodymium to make lenses for glass maker’s goggles since it filters out the yellow light present in glass blowing. Alloyed with magnesium creates a high-strength metal used in aircraft engines. Misch metal, used in the manufacture of pyrophoric alloys for cigarette lighters, contains about 5 % praseodymium metal. (Typically composition of misch metal are Ce:Nd:Pr:La:Other rare earth=50:18:6:22:4).
refractometer → refraktometar
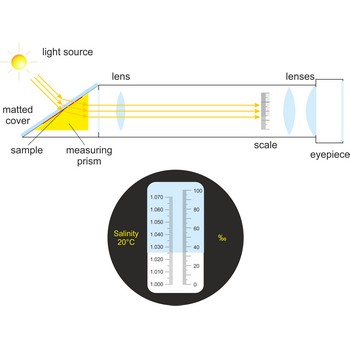
Refractometer is an optical device used from measurement of refractive index. A refractometer takes advantage of the fact that light bends as it passes through different materials. It can be used to measure the salinity of water or the amount of sugar in fresh grapes. Refractometers are available with or without automatic temperature compensation (ATC).
When using a conventional saltwater refractometer, a sample is placed on an optical prism in the sample window. As light shines through the sample, it is bent according to the salinity of the water, and casts a shadow on the scale that is visible through the eyepiece. Salinity is read directly through the eyepiece.
seawater → more
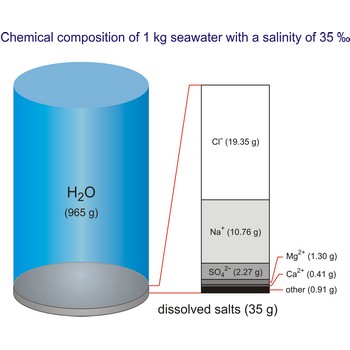
Seawater is a complex mixture of 96.5 % water, 3.5 % salts, and smaller amounts of other substances, including dissolved inorganic and organic materials, particulates, and a few atmospheric gases. The world's oceans cover nearly 71 % (361 840 000 km2) of the Earth's surface (510 100 000 km2), with an average depth of 3 682.2 m.
The density of seawater is higher than that of fresh water because of its higher salinity. Seawater's freezing point is lower than that of pure water and its boiling point is higher. The average salinity of the ocean is 35 ‰, which means that for every kilograms of water, there are 35 g of salt. The relative abundance of the major salts in seawater are constant regardless of the ocean. Only six elements and compounds comprise about 99 % of sea salts: chlorine (Cl-), sodium (Na+), sulfur (SO42-), magnesium (Mg2+), calcium (Ca2+), and potassium (K+).
sodium → natrij
Sodium was discovered by Sir Humphry Davy (England) in 1807. The origin of the name comes from the Latin word natrium meaning sodium carbonate. It is soft silvery-white metal. Fresh surfaces oxidize rapidly. Reacts vigorously, even violently with water. Reacts with water to give off flammable gas. Burns in air with a brilliant white flame. Sodium is obtained by electrolysis of melted sodium chloride (salt), borax and cryolite. Metallic sodium is vital in the manufacture of organic compounds. Sodium chloride (NaCl) is table salt. Liquid sodium is used to cool nuclear reactors.
starch → škrob
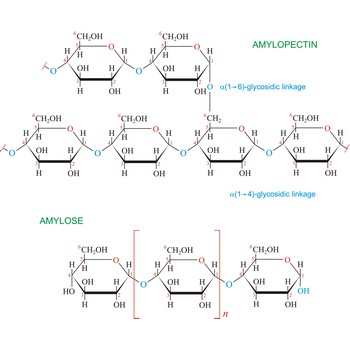
Starch (C6H10O5)x is a polysaccharide used by plants to stockpile glucose molecules. It is the major component of flour, potatoes, rice, beans, corn, and peas. Starch is a mixture of two different polysaccharides: amylose (about 20 %), which is insoluble in cold water, and amylopectin (about 80 %), which is soluble in cold water. Amylose is composed of unbranched chains of D-glucose units joined by α(1→4)-glycosidic linkages. Unlike amylose, which are linear polymers, amylopectin contains α(1→6)-glycoside branches approximately every 25 glucose units.
Starch digestion begins in the mouth via the action of amylase, a digestive enzyme present in saliva. The process is completed in the small intestine by the pancreatic amylase. The final products of starch digestion, glucose molecules, are absorbed into the intestinal bloodstream and transported to the liver. Like most enzymes, glycosidases are highly selective in their action. They hydrolyze only the α-glycoside links in starch and leave the β-glycoside links in cellulose untouched. Starch is important food stuff and is used in adhesives, and sizes, in laundering, pharmacy and medicine.
Citing this page:
Generalic, Eni. "With a pinch of salt." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table