electronegativity → elektronegativnost
Electronegativity is a parameter originally introduced by L. Pauling which describes, on a relative basis, the power of an atom to attract electrons. For example, in hydrogen chloride, the chlorine atom is more electronegative than the hydrogen and the molecule is polar, with a negative charge on the chlorine atom.
There are various ways of assigning values for the electronegativity of an element. Pauling electronegativities are based on bond dissociation energies using a scale in which fluorine, the most electronegative element, has the value 4 and francium, the lowest electronegative element, has the value 0.7.
rate equation → jednadžba brzine reakcije
Rate equation is an equation that describes the dependence of reaction rate on concentrations of reacting species. It always has the form
where a and b are usually integral exponents.
salt fog chambers → slana komora
Salt fog chambers are designed for corrosive atmosphere testing. The samples being tested are inserted into the chamber and then the salt-containing solution is sprayed as a very fine fog mist over the samples. The temperature within the chamber is maintained constant (usually 35 °C). These test chambers are constructed of non-corrosive materials.
theories of catalysis → teorije katalize
Theories of catalysis explain the influence of the catalysts upon the rate of a reaction by describing the detailed mechanism by which the catalyst is involved in the steps of the chemical reaction.
electron → elektron
The electron is an elementary particle with a negative electric charge of (1.602 189 2±0.000 004 6)×10-19 C and a mass of 1/1837 that of a proton, equivalent to (9.109 534±0.000 047)×10-31 kg.
In 1897 the British physicist Joseph John (J.J.) Thomson (1856-1940) discovered the electron in a series of experiments designed to study the nature of electric discharge in a high-vacuum cathode-ray tube. Thomson interpreted the deflection of the rays by electrically charged plates and magnets as evidence of bodies much smaller than atoms that he calculated as having a very large value for the charge to mass ratio. Later he estimated the value of the charge itself.
Electrons are arranged in from one to seven shells around the nucleus; the maximum number of electrons in each shell is strictly limited by the laws of physics (2n2). The outer shells are not always filled: sodium has two electrons in the first shell (2×12 = 2), eight in the second (2×22 = 8), and only one in the third (2×32 = 18). A single electron in the outer shell may be attracted into an incomplete shell of another element, leaving the original atom with a net positive charge. Valence electrons are those that can be captured by or shared with another atom.
Electrons can be removed from the atoms by heat, light, electric energy, or bombardment with high-energy particles. Decaying radioactive nuclei spontaneously emit free electrons, called β particles.
Gauss’ law for electrostatics → Gaussov zakon za elektrostatiku
Gauss’ law describes the relation between charge and electric field in static situations, so it is equivalent to Coulomb’s law, which can be derived from Gauss’ law. Gauss’ law states that the net flux of electric field, Φ, through an imaginary closed surface, S, - a Gaussian surface - is equal to the net charge, q, inside that closed surface:
where electric flux Φ through Gaussian surface is given by:
ε0 is the permittivity constant and dS is a surface element.
Geiger counter → Geigerov brojač
Geiger counter (Geiger-Muller counter) is a device used to detect and measure ionising radiation. It consists of a tube containing a low-pressure gas (usually argon or neon with methane) and a cylindrical hollow cathode through the centre of which runs a fine-wire anode. A potential difference of about 1 000 V is maintained between the electrodes. An ionising particle or photon passing through a window into the tube will cause an ion to be produced and the high potential will accelerate it towards its appropriate electrode, causing an avalanche of further ionisations by collision. The consequent current pulses can be counted in electronic circuits or simply amplified to work a small loudspeaker in the instrument. It was first devised in 1908 by the German physicist Hans Geiger (1882-1945). Geiger and W. Muller produced an improved design in 1928.
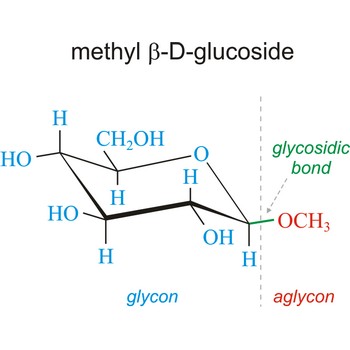
glycosidic bond → glikozidna veza
Glycosidic bond ia a bond between the glycosyl group, the structure obtained by removing the hydroxy group from the hemiacetal function of a monosaccharide, and the -OR group (which itself may be derived from a saccharide and chalcogen replacements thereof (RS–, RSe–). The terms N-glycosides and C-glycosides are misnomers and should not be used. The glycosidic bond can be α or β in orientation, depending on whether the anomeric hydroxyl group was α or β before the glycosidic bond was formed and on the specificity of the enzymatic reaction catalyzing their formation. Once the glycosidic bond is formed, the anomeric configuration of the ring is locked as either α or β. Specific glycosidic bonds therefore may be designated α(1→4), β(1→4), α(1→6), and so on. Cellulose is formed of glucose molecules linked by β(1→4)-glycosidic bonds, whereas starch is composed of α(1→4)-glycosidic bonds.
Citing this page:
Generalic, Eni. "What are desi folks." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table