desiccant dryers → sredstva za sušenje
Desiccant dryers (air dryers) use nonconsumable chemicals such as silica gel or active alumina, and can remove almost all the moisture from compressed air by adsorbing moisture on its surface. The method of regeneration, the process of removing adsorbed water from the desiccant, is the primary distinguishing feature among the various types of desiccant dryers. There are three ways to regenerate a desiccant: with air, internal or external heaters, or a heat pump.
desiccator → eksikator
Desiccator is a glass container with dry atmosphere due to the presence of some dehydrating agent. It is used for protecting the samples, reagents or precipitates from humidity. As dehydrating agent usually waterless calcium chloride (CaCl2) is used.
accelerated corrosion test → ubrzana korozija
Accelerated corrosion test is method designed to approximate, in a short time, the deteriorating effect under normal long-term service conditions.
allotropy → alotropija
Allotropy (Gr. allos, other, and tropos, manner) is the phenomenon of an element existing in two or more physical forms in the same physical state. The difference between the forms involves either crystaline structure (white, red and black phosphorus), the number of atoms in the molecule of a gas (diatomic oxygen and triatomic ozone), or the molecular structure of a liquid (liquid helium an helium II).
In some cases, the allotropes are stable over a temperature range, with a definite transition point at which one changes into the other. For instance, tin has two allotropes: white (metallic) tin stable above 13.2 °C and grey (nonmetallic) tin stable below 13.2 °C. This form allotropy is called enantiotropy. Form of allotropy, in which there is no transition temperature at which the two are in equilibrium, is called monotropy.
Allotropy does not apply to the substance existing in different physical states as, for example, when ice melts and changes from solid ice to liquid water.
Allotropy is generally restricted to describing polymorphic behaviour in elements, while polymorphism may refer to any material having multiple crystal structures.
anomer → anomer
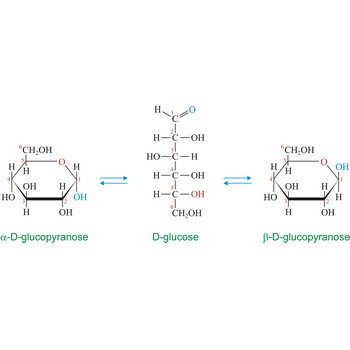
Anomers are diastereoisomers of cyclic forms of sugars or similar molecules differing in the configuration at the anomeric carbon (C-1 atom of an aldose or the C-2 atom of a 2-ketose). The cyclic forms of carbohydrates can exist in two forms, α- and β- based on the position of the substituent at the anomeric center. Anomer are designated α if the configuration at the anomeric carbon is the same as that at the reference asymmetric carbon in a Fischer projection. If the configuration differs the anomer is designated β. For example, α-D-glucopyranose and β-D-glucopyranose, the two cyclic forms of glucose, are anomers.
antioxidant → antioksidans
Antioxidants are compounds that slow down oxidation processes that degrade foods, fuels, rubber, plastic, and other materials. Antioxidants like butylated hydroxyanisole (BHA), are added to food to prevent fats from becoming rancid and to minimize decomposition of vitamins and essential fatty acids; they work by scavenging destructive free radicals from the food.
aqueous solution → vodena otopina
Aqueous solutions are those solutions where water is the solvent. An aqueous solution found in an equation describing a chemical reaction is denoted by the state symbol, (aq).
astronomical unit → astronomska jedinica
Astronomical unit (AU) is a unit of length employed in astronomy for describing planetary distance. It is the mean distance of the earth from the sun, equal to 1.49597870×1011 m.
Citing this page:
Generalic, Eni. "What are desi folks." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table